Carbon dioxide and water react to form methanol and oxygen, like this: 2 CO,(9)+4 H,0(9)→2 CH,OH()+3 O,(9) At a certain temperature, a chemist finds that a 4.6 L reaction vessel containing a mixture of carbon dioxide, water, methanol, and axygen at equilibrium has the following composition: compound amount CO, 3.94 g H,0 4.50 g CH,OH 3.68 g 2.67 8 Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ - 0 Explanation Check 2022 MeGraw H LLC A Rights Reserved Terms of Use Pvacy Center Accessibty pe here to search (99+
Carbon dioxide and water react to form methanol and oxygen, like this: 2 CO,(9)+4 H,0(9)→2 CH,OH()+3 O,(9) At a certain temperature, a chemist finds that a 4.6 L reaction vessel containing a mixture of carbon dioxide, water, methanol, and axygen at equilibrium has the following composition: compound amount CO, 3.94 g H,0 4.50 g CH,OH 3.68 g 2.67 8 Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ - 0 Explanation Check 2022 MeGraw H LLC A Rights Reserved Terms of Use Pvacy Center Accessibty pe here to search (99+
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 3RQ
Related questions
Question
![Carbon dioxide and water react to form methanol and oxygen, like this:
2 CO,(g)+4 H,O(g)→2CH;OH(1)+3 0,(9)
At a certain temperature, a chemist finds that a 4.6 L reaction vessel containing a mixture of carbon dioxide, water, methanol, and oxygen at equilibrium has
the following composition:
compound amount
CO,
3.94 g
H,0
4.50 g
CH;OH
3.68 g
2.67 g
Calculate the value of the equilibrium constant K. for this reaction. Round your answer to 2 significant digits.
K_ = ]
Explanation
Check
O 2022 McGraw H LLC AI Rights Reserved Terms of Use
ype here to search
99+
B
Alt](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd9a2776d-a528-4478-be0e-235d689690c2%2F0d6a73dc-9300-4600-96e8-5778015a9352%2Fbl7z9ej_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Carbon dioxide and water react to form methanol and oxygen, like this:
2 CO,(g)+4 H,O(g)→2CH;OH(1)+3 0,(9)
At a certain temperature, a chemist finds that a 4.6 L reaction vessel containing a mixture of carbon dioxide, water, methanol, and oxygen at equilibrium has
the following composition:
compound amount
CO,
3.94 g
H,0
4.50 g
CH;OH
3.68 g
2.67 g
Calculate the value of the equilibrium constant K. for this reaction. Round your answer to 2 significant digits.
K_ = ]
Explanation
Check
O 2022 McGraw H LLC AI Rights Reserved Terms of Use
ype here to search
99+
B
Alt
Expert Solution
Step 1
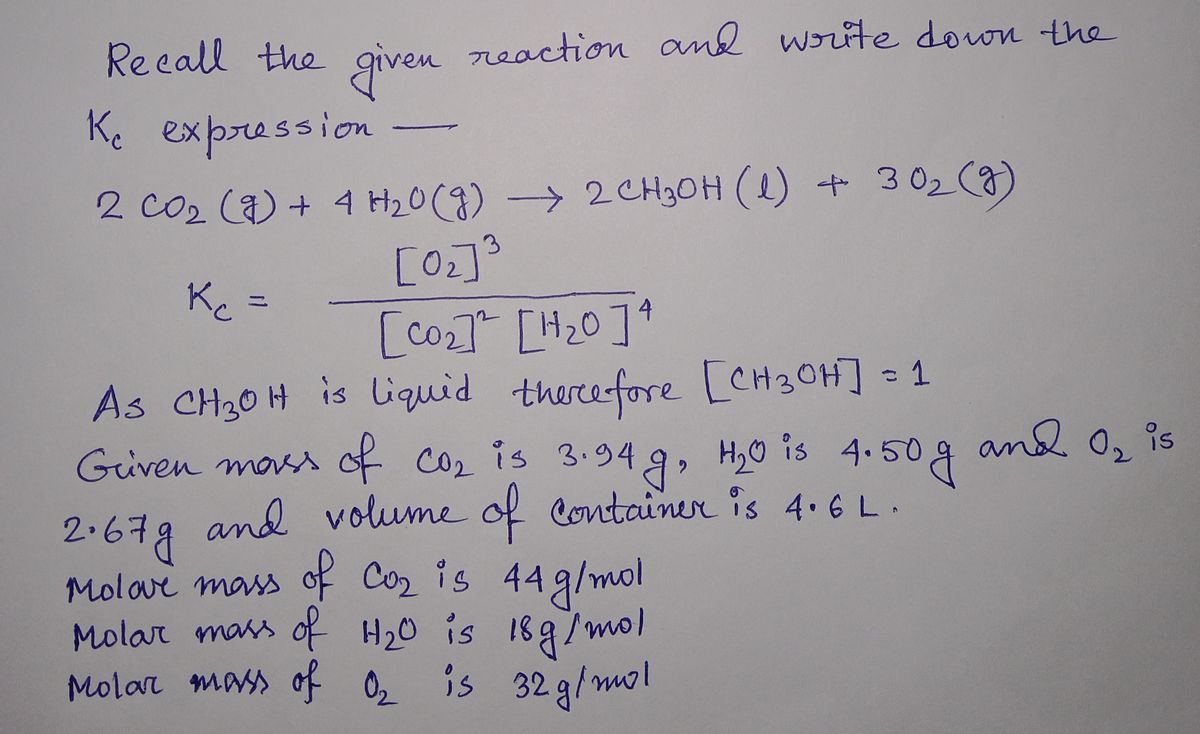
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning