Carbohydrates
Carbohydrates are the organic compounds that are obtained in foods and living matters in the shape of sugars, cellulose, and starch. The general formula of carbohydrates is Cn(H2O)2. The ratio of H and O present in carbohydrates is identical to water.
Starch
Starch is a polysaccharide carbohydrate that belongs to the category of polysaccharide carbohydrates.
Mutarotation
The rotation of a particular structure of the chiral compound because of the epimerization is called mutarotation. It is the repercussion of the ring chain tautomerism. In terms of glucose, this can be defined as the modification in the equilibrium of the α- and β- glucose anomers upon its dissolution in the solvent water. This process is usually seen in the chemistry of carbohydrates.
L Sugar
A chemical compound that is represented with a molecular formula C6H12O6 is called L-(-) sugar. At the carbon’s 5th position, the hydroxyl group is placed to the compound’s left and therefore the sugar is represented as L(-)-sugar. It is capable of rotating the polarized light’s plane in the direction anticlockwise. L isomers are one of the 2 isomers formed by the configurational stereochemistry of the carbohydrates.
Draw a Fischer projection of the monosaccharide from which each of the
following glycosides was prepared.

Monosaccharides are the simple sugars, which cannot be hydrolyzed further into simpler forms and they have a general formula here, n is the number of carbon atoms. Some common examples are glucose, ribose, fructose, etc.
A Fischer projection is represented by the cross (+), where horizontal line represents the bonds are above the plane and vertical line represents the bonds are below the plane. The point of intersection of two lines (vertical and horizontal lines) represents the chiral carbon that lies in the plane of paper.
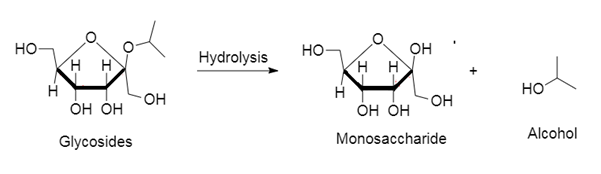
Glycosides: It is a compound in which one or more sugars are combined together with non-sugar molecules via glycosidic linkage.
Glycosides on hydrolysis yield the corresponding monosaccharide and alcohol. The reaction will be:
Step by step
Solved in 3 steps with 2 images









