The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E,=6.0 kJ/mol. If the rate constant of this -1-1 reaction is 2.2 x 10% M 's at 208.0 °C, what will the rate constant be at 117.0 °C? Round your answer to 2 significant digits.
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E,=6.0 kJ/mol. If the rate constant of this -1-1 reaction is 2.2 x 10% M 's at 208.0 °C, what will the rate constant be at 117.0 °C? Round your answer to 2 significant digits.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.58PAE
Related questions
Question
100%
Use the Arrhenius equation to find k at one temperature vs another.

Transcribed Image Text:The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E,=6.0 kJ/mol. If the rate constant of this
reaction is 2,2 x 10%
-1-1
M 's
at 208.0 °C, what will the rate constant be at 117.0 °C?
Round your answer to 2 significant digits.
-1
k =
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit
8:33
Type here to search
29°F Cloudy
2/16/
DELL
F12
PrtScr
Insert
Delete
Pgup
ンニン
F3
F4
F5
F6
F7
F8
F9
F10
F11
Num
Loc
$
&
Backspace
Expert Solution
Step 1
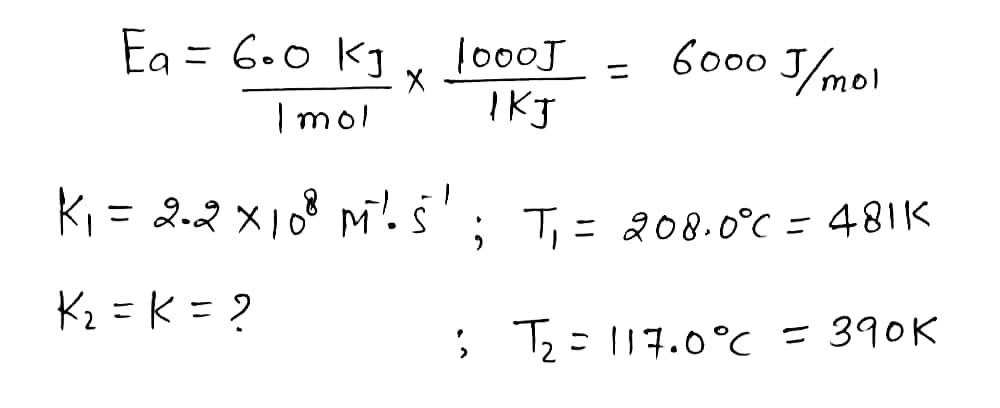
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning