During a glucose tolerance test, the serum glucose concentration of a patient was found to be 131 mg/dL. Convert the concentration to grams per liter.
During a glucose tolerance test, the serum glucose concentration of a patient was found to be 131 mg/dL. Convert the concentration to grams per liter.
Chapter12: The Liquids And Solids Around Us: Especially Water
Section: Chapter Questions
Problem 47E
Related questions
Question
During a glucose tolerance test, the serum glucose concentration of a patient was found to be 131 mg/dL. Convert the concentration to grams per liter.
Expert Solution
Step 1
The first step is to determine single conversion factors. To determine a single factor for unit conversion, we have to know the relationship between the two units. Once we know the relationship, the factor can be derived easily. This is done by placing the unit to be converted in the denominator of the factor so that it cancels out.
From Table 1.3, we have the following conversion factors:
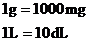
Thus, the conversion factors needed are 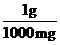 and
and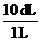
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning