
Concept explainers
a.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangement of atoms in the molecule are said to be isomers of each other.
a.
Answer to Problem 32PP
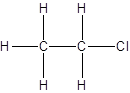
Explanation of Solution
For the first isomer structure, all two-carbon atoms are written in a straight chain and the chlorine atom is bonded to one of the carbon atoms as:
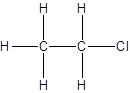
There is no other possibility for the different structural formula
b.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangement of atoms in the molecule are said to be isomers of each other.
b.
Answer to Problem 32PP
Isomer I:
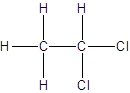
Isomer II:
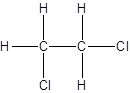
Explanation of Solution
The different structural the arrangement of atoms in the molecule
- All two-carbon atoms are written in a straight chain and two chlorine atoms are bonded to one of the carbon atoms resulting in:
- Interchanging the position of one hydrogen atom at first carbon with one chlorine atom of the second carbon atom.
Isomer I:
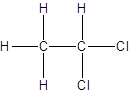
Isomer II:
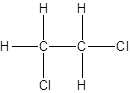
c.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangements of atoms in the molecule are said to be isomers of each other.
c.
Answer to Problem 32PP
Isomer I:
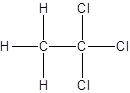
Isomer II:
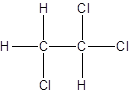
Explanation of Solution
The different structural arrangement of atoms in the molecule
- All two-carbon atoms are written in a straight chain and three chlorine atoms are bonded to one of the carbon atoms resulting in:
- Interchanging the position of one hydrogen atom at first carbon with one chlorine atom of the second carbon atom.
Isomer I:
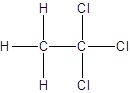
Isomer II:
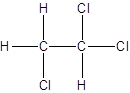
d.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangements of atoms in the molecule are said to be isomers of each other.
d.
Answer to Problem 32PP
Isomer I:
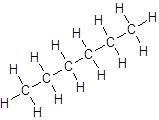
Isomer II:
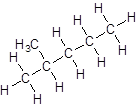
Isomer III:
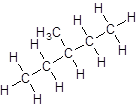
Isomer IV:
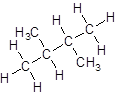
Isomer V:
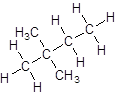
Explanation of Solution
The different structural arrangement of atoms in the molecule
- All six-carbon atoms are written in a straight chain resulting in:
- Five-carbon atoms are written in a straight chain and a methyl group is attached to the second carbon resulting in:
- Five-carbon atoms are written in a straight chain and a methyl group is attached to the third carbon resulting in:
- Four-carbon atoms are written in a straight chain and a methyl group is attached to the second and third carbon resulting in:
- Four-carbon atoms are written in a straight chain and two methyl groups are attached to the second carbon resulting in:
Isomer I:
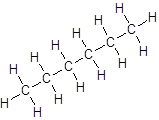
Isomer II:
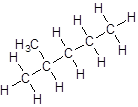
Isomer III:
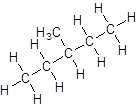
Isomer IV:
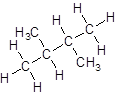
Isomer V:
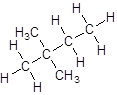
Want to see more full solutions like this?
Chapter 1 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





