
(a)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 42E
Electron dot structure and structural formula of
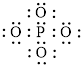
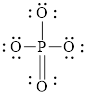
Explanation of Solution
In molecule
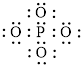
Figure 1

Figure 2
Solid line, in Figure 2, between the phosphorous and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the atoms present in that bond.
An electron dot structure and structural formula of
(b)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 42E
Electron dot structure and structural formula of
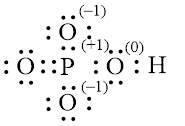
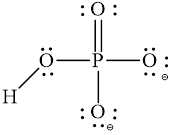
Explanation of Solution
In molecule
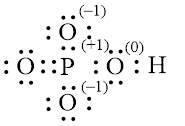
Figure 3

Figure 4
Each solid line, in Figure 4, between the phosphorous and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom phosphorous and the surrounding oxygen atoms.
An electron dot structure and structural formula of
(c)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 42E
Electron dot structure and structural formula of
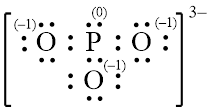
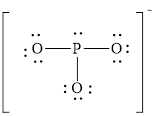
Explanation of Solution
In molecule
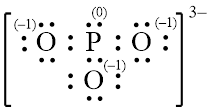
Figure 5

Figure 6
Each solid line, in Figure 6, between the phosphorous and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom and the surrounding atom.
An electron dot structure and structural formula of
(d)
Interpretation:
The electron dot formula and structural formula of
Concept introduction:
An electron dot formula is a way of representing the molecular structure in which electrons are represented by a dot. Structural formula is a way in which atoms are linked together through a solid line. This solid line represents the covalent bond. An electron dot structure is known as Lewis structure. Electron dot structure indicates the valence electrons of an atom which are involved in bonding.
Answer to Problem 42E
Electron dot structure and structural formula of
![]()
Explanation of Solution
In molecule
![]()
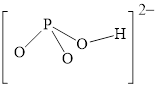
Figure 7

Figure 8
Each solid line, in Figure 8, between the phosphorous and oxygen atom is the covalent bond which is made up of two electrons. This bond is formed by sharing of electrons between the central atom and the surrounding atom.
An electron dot structure and structural formula of
Want to see more full solutions like this?
Chapter 12 Solutions
Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- Write formulas for the compounds:(i) iodic acidarrow_forwardWrite the chemical formulas for the following compounds:(a) Silver cyanide(b) Calcium hypochlorite(c) Potassium chromate(d) Gallium oxide(e) Potassium superoxide(f) Barium hydrogen carbonatearrow_forwardWhich of the following statements are true regarding a covalent bond between nitrogen and oxygen atoms in a nitric oxide, NO, molecule? (a) Valence electrons are shared between nitrogen and oxygen atoms. (b) Bonding electrons are found only between the bonded atoms. (c) The bond length is greater than the sum of the two atomic radii. (d) Energy is required to break a covalent bond.arrow_forward
- Predict which of these compounds are ionic and which are covalent.(A) Ca3N2(B) Li2CO3(C) PCl5(D) NaOH(E) CH4(F) MgOarrow_forwardAnswer true or false. (a) The name of a binary ionic compound consists of the name of the positive ion followed by the name of the negative ion. (b) In naming binary ionic compounds, it is necessary to state the number of each ion present in the compound. (c) The formula of aluminum oxide is Al2 O3 . (d) Both copper(II) oxide and cupric oxide are acceptable names for CuO. (e) The systematic name for Fe2 O3 is iron(II) oxide. (f) The systematic name for FeCO3 is iron carbonate. (g) The systematic name for NaH2PO4 is sodium di- hydrogen phosphate. (h) The systematic name for K2HPO4 is dipotassium hydrogen phosphate. (i) The systematic name for Na2O is sodium oxide. (j) The systematic name for PCl3 is potassium chloride. (k) The formula of ammonium carbonate is NH4CO3. 39. (a) A covalent bond is formed between two atoms whose difference in electronegativity is less than 1.9. (b) If the difference in electronegativity between two atoms is zero (they have identical electronegativ- ities),…arrow_forwardThe atomic number of sulfur is 16. Sulfur combines withhydrogen by covalent bonding to form a compound, hydrogensulfide. Based on the number of valence electrons in a sulfuratom, predict the molecular formula of the compound.(A) HS(B) HS2(C) H2S(D) H4Sarrow_forward
- Use your knowledge of the correct number of covalent bonds to predict the formula for a simple compound formed between bromine and the following elements. (Enter NONE if no compound is likely to form.) (a) phosphorus (b) carbon (c) nitrogen (d) hydrogenarrow_forwardWrite the formulas of the following covalent compounds. (Type your answer using the format CO2 for CO2.) (a) silicon tetrachloride(b) carbon tetrachloride(c) dinitrogen tetroxide(d) dinitrogen oxidearrow_forwardWrite a Lewis structure for each of the following molecules or ions. (a) H3BPH3(b) BF4−(c) BBr3(d) B(CH3)3(e) B(OH)3arrow_forward
- Which statement about the properties of barium chloric and mercury(II) chloride is correct? (A) BaCl₂ has a higher melting point than HgCl₂. (B) BaCl₂ has a higher solubility in nonpolar solvents than HgCl₂. (C) BaCl₂ has a higher vapor pressure than HgCl₂. (D) Molten BaCl₂ has a lower electrical conductivity than molten HgCl2.arrow_forwardIllustrated are four ions — A, B, X, and Y— showing their relative ionic radii. The ions shown in red carry positive charges: a 2+ charge for A and a 1+ charge for B. Ions shown in blue carry negative charges: a 1- charge for X and a 2- charge for Y. (a) Which combinations of these ions produce ionic compounds where there is a 1:1 ratio of cations and anions?(b) Among the combinations in part (a), which leads to the ionic compound having the largest lattice energy? Why?arrow_forwardElements in the same group of the periodic table often formoxyanions with the same general formula. The anions arealso named in a similar fashion. Based on these observations,suggest a chemical formula or name, as appropriate, for eachof the following ions: (a) BrO4-, (b) SeO32-, (c) arsenate ion,(d) hydrogen tellurate ion.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





