
Concept explainers
Interpretation:
The structural formula corresponding to the given name has to be stated.
Concept Introduction:
The systematic naming of organic compound is given by
Rules for writing structural formula from IUPAC are as follows:
First identify the word root for the given compound.
Identify the suffix used in the given compound like –ene, oic, ane, etc.
Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 13P
Solution:
a) The structural formula corresponding to the given name is shown below.
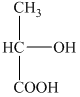
b) The structural formula corresponding to the given name is shown below.
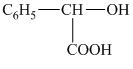
c) The structural formula corresponding to the given name is
d) The structural formula corresponding to the given name is
e) The structural formula corresponding to the given name is shown below.
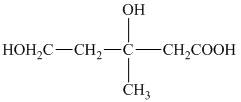
f) The structural formula corresponding to the given name is shown below.
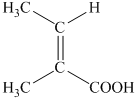
g) The structural formula corresponding to the given name is shown below.
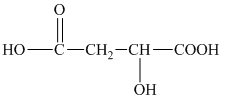
h) The structural formula corresponding to the given name is shown below.
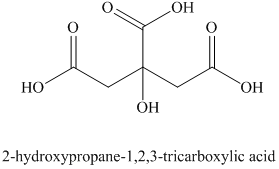
i) The structural formula corresponding to the given name is shown below.
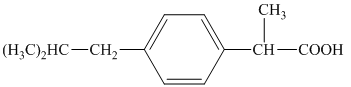
j) The structural formula corresponding to the given name is shown below.
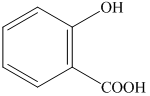
Explanation of Solution
a) Structural formula of
The given name is
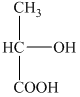
b) Structural formula of
The given name is
c) Structural formula of tetradecanoic acid.
The given name is tetradecanoic acid. The word root used in this is tetradec. It means structure contains fourteen carbon atoms. The functional group present in the given compound is carboxylic acid
d) Structural formula of
The given name is
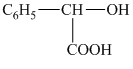
e) Structural formula of
The given name is
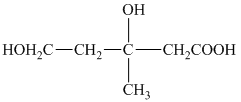
f) Structural formula of
The given name is
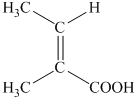
g) Structural formula of
The given name is
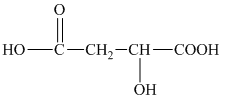
h) Structural formula of
The given name is
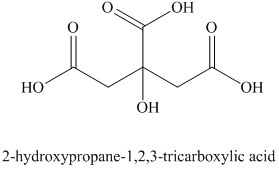
i) Structural formula of
The given name is
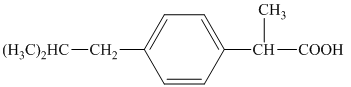
j) Structural formula of
The given name is
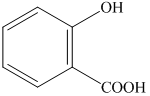
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry - Standalone book
- The foul odor of rancid butter is caused by butyric acid, CH3CH2CH2CO2H.(a) Draw the Lewis structure and determine the oxidation number and hybridization for each carbon atom in the molecule.(b) The esters formed from butyric acid are pleasant-smelling compounds found in fruits and used in perfumes. Draw the Lewis structure for the ester formed from the reaction of butyric acid with 2-propanol.arrow_forwardWrite structures and describe thephysical properties of carboxylic acidsarrow_forwardCompounds that contain an N-H group associate by hydrogen bonding. (a) Do you expect this association to be stronger or weaker than that of compounds containing an O-H group? (b) Based on your answer to part (a), which would you predict to have the higher boiling point, 1-butanol or 1-butanamine?arrow_forward
- (i) Draw the structure of any amine and give the IUPAC name of that amine. (1) Classify the amine in your answer provided in (i) above (iii) Draw the structure of ethyl butanoate and name the functional group. (iv) Give the IUPAC name of the following compound and name the functional group:arrow_forwardGive the structural formulae and name the functional groups of the following compounds. (a) 3-chlorobut-1-ene Name the functional group: (b) butanedioic acid Name the functional group: (c) propanamide Name the functional group: (d) 3-methylbutanal Name the functional group:arrow_forwardExplain the geometry,hybridization and bond angle of carbon atom of a carboxylic acid?arrow_forward
- Draw Structural formulas to the eight carboxylic acids with the molecular formula C6H12O2.arrow_forwardPlease refer to the molecule shown below when answering questions: (a) Depending on the reagent used, which functional group is capable of undergoing E1/E2or SN1/SN2 reactions?(b) An aqueous solution of this functional group is likely to turn blue litmus paper red:(c) Identify a functional group that can easily undergo addition reactions with HBr or HCl.(d) Identify a secondary amide.arrow_forwardThe normal pH range for blood plasma is 7.35–7.45. Under these conditions, would you expect the carboxyl group of lactic acid (pKa 3.08) to exist primarily as a carboxyl group or as a carboxylic anion? Explain.arrow_forward
- Ester compounds often have a sweet, pleasant odor. Many characteristic fruit scents are largely due to the natural presence of one or more ester compounds. As such, artificial scents for foods are often composed of complex mixtures of various esters. The exact identity and ratio of ingredients that compose a particular scent are closely guarded secrets in the food and fragrance industry. Suppose that you are a chemist working for a company that i creating a new line of air fresheners. The company is considering three scents: apple, pear, and pineapple. The project manager has asked you to prepare the ester compounds that are largely responsible for these scents. The structural formulas for these ester compounds are shown here: Alcohols for Air Freshener Project Molar mass Density Cost, per (g/mL) Reagent (g/mol) 1.00 L methanol 32.04 0.79 $46.20 ethanol 46.07 0.79 $112.00 1-propanol 60.10 0.80 $72.70 1-butanol 74.12 0.81 $72.60 Use the structural formulas of the alcohols and…arrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardDoes methanol behave as an acid or a base when it reacts with methylamine?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,  Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning




