
a)
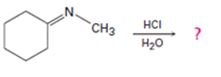
Interpretation:
The products formed in the reaction given are to be identified. The mechanism of the reaction also is to be provided.
Concept introduction:
The protonation of the imine yields a iminium ion. The nucleophilc attack by water followed by exchange of proton from O to N and elimination of a proton will yield an
To identify:
The products formed in the reaction given and to provide the mechanism of the reaction.
b)
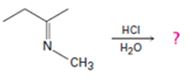
Interpretation:
The products formed in the reaction given are to be identified. The mechanism of the reaction also is to be provided.
Concept introduction:
The protonation of the imine yields a iminium ion. The nucleophilc attack by water followed by exchange of proton from O to N and elimination of a proton will yield an amine and an aldehyde or ketone as the product.
To identify:
The products formed in the reaction given and to provide the mechanism of the reaction.
c)
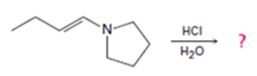
Interpretation:
The products formed in the reaction given are to be identified. The mechanism of the reaction also is to be provided.
Concept introduction:
The protonation of the imine yields a iminium ion. The nucleophilc attack by water followed by exchange of proton from O to N and elimination of a proton will yield an amine and an aldehyde or ketone as the product.
To identify:
The products formed in the reaction given and to provide the mechanism of the reaction.
d)
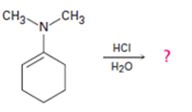
Interpretation:
The products formed in the reaction given are to be identified. The mechanism of the reaction also is to be provided.
Concept introduction:
The protonation of the imine yields a iminium ion. The nucleophilc attack by water followed by exchange of proton from O to N and elimination of a proton will yield an amine and an aldehyde or ketone as the product.
To identify:
The products formed in the reaction given and to provide the mechanism of the reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry
- Predict the product(s) and provide the mechanism for each reaction below.arrow_forward: Treatment of (CHa)CHCH(OH)CH,CH3 with TSOH affords two products (M and N) with molecular formula CgH12. The 'H NMR spectra of M and N are given below. Propose structures for M and N and draw a mechanism to explain their formation. 1H NMR of M 3H 1H NMR of N 3H 3H 3 H 1H 3 H 2 H 2H 2H 8 7 6 4 1 0 9 8. 2 1 ppm ppm 4.arrow_forwardTreatment of (CH3)2CHCH(OH)CH2CH3 with TsOH affords two products (M and N) with molecular formula C6H12. The 1H NMR spectra of M and N are given below. Propose structures for M and N and draw a mechanism to explain their formation.arrow_forward
