
Tutorials in Introductory Physics
1st Edition
ISBN: 9780130970695
Author: Peter S. Shaffer, Lillian C. McDermott
Publisher: Addison Wesley
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 27.2, Problem 2aTH
One mole of an ideal gas is confined to a container with a movable piston. The questions below refer to the processes shown on the PV diagram at right. Process I is a change front state X to state Y at constant pressure. Process II is a change from state W to state Z at a different constant pressure.
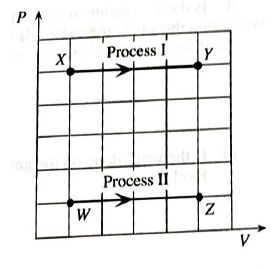
- Rank the temperatures of states W, X, Y, and Z. If any temperatures are equal, state that explicitly. Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Suppose a monatomic ideal gas is changed from state A to state D by one of the processes shown on the PV diagram.
1. The gas follows the constant-temperature path AC followed by the constant-pressure path CD.
What is the total work done on the gas ?
What is the total change in internal energy of the gas during the entire process?
What is the total heat flow into the gas?
Problem 2
Suppose an irreversible process takes 1.0 mol of
monoatomic ideal gas from state 1 to state 2, where
pressure increases linearly with volume as shown in the
diagram below.
P2
Suppose
that
the following
measurements are taken:
1
P1
Pi=101,000 N/m?
Vi = 0.010 m³
p2=185,000 N/m?
V2 = 0.026 m
V1
V2
Calculate the following:
a) work done on the system for the process.
b) heat into the system for the process.
c) AUsys for going from state 1 to 2. Give your answer in units of joules (J).
d) ASsys for going from state 1 to 2. Give your answer in units of J/K.
e) What can be known about ASsurr for this process?
V (m³)
p (N/m²)
Part B
A container holds a sample of ideal gas in thermal equilibrium, as shown in the
figure. (Figure 1) One end of the container is sealed with a piston whose head is
perfectly free to move, unless it is locked in place. The walls of the container readily
allow the transfer of energy via heat, unless the piston is insulated from its
surroundings.
Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process?
"Immerse the container into a large water bath at the same temperature, and very slowly push the piston head further into the container."
• View Available Hint(s)
O point 1
O point 2
O point 3
O point 4
O point 5
O point 6
O point 7
O point 8
Submit
Previous Answers
X Incorrect; Try Again; 4 attempts remaining
The volume of the gas cannot remain constant because the piston head has moved further into the container.
Part C
Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas…
Chapter 27 Solutions
Tutorials in Introductory Physics
Ch. 27.1 - Prob. 1aTHCh. 27.1 - In this process, which of the quantities P, V, n,...Ch. 27.1 - Consider the following incorrect student...Ch. 27.1 - Explain why it is not possible to use the ideal...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - Prob. 2cTHCh. 27.2 - Prob. 1aTHCh. 27.2 - Prob. 1bTHCh. 27.2 - Prob. 1cTH
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why are the seasons on Uranus different from the seasons on any other planet?
Conceptual Integrated Science
3. What is free-fall, and why does it make you weightless? Briefly describe why astronauts are weightless in th...
The Cosmic Perspective
32. Water flowing through a 2.0-cm-diameter pipe can fill a 300 L bathtub in 5.0 min. What is the speed of the ...
College Physics: A Strategic Approach (4th Edition)
Rooms A and B are the same size, and are connected by an open door. Room A, however, is warmer (perhaps because...
An Introduction to Thermal Physics
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- First Law of Thermodynamics PROCEDURES: in the pictures attached A.When gas expands, Is the (W) work done by the system or (W) work done on the system? Explain your answer. B.Compare the temperature outside (surroundings) and inside (system) the bottle before pouring the boiling water into the bucket. C.Compare the temperature outside (surroundings) and inside (system) the bottle after pouring the boiling water into the bucket.arrow_forwardA gas undergoes the process shown in the diagram below. During the process AB, the internal energy of the gas decreases and a certain amount of heat Q goes out of the system for the process CA. Use this information to answer the questions below. Options for each selection is Positive, Negative, or Zero (check attached image) (a) What are the signs of W (work done by the gas), Q, and ΔU for the process CA?(b) What are the signs of W (work done by the gas), Q, and ΔU for the process AB?(c) What are the signs of W (work done by the gas), Q, and ΔU for the process BC?arrow_forwardReferring to the figure shown, water contained in a piston–cylinder assembly, initially at 100 kPa and 25°C. The water is heated until the final temperature reaches to 320°C. Kinetic and potential energy effects are negligible.Sketch the process on T-v diagram. Determinea. The quality of the mixture when piston hits the stop.b. The final pressure.Hint: Establish the problem in three states; 1: initial, 2: when piston hits the stop, 3: at T3 = 320°C.arrow_forward
- Give an equation for the infinitesimal change in entropy, ??, of a system in terms of the heat transfer ??? and the temperature ? at which the heat transfer occurs. Explain the sign convention.ii. A litre of water at 20 °C is placed in a fridge at 5 °C. Calculate the change in entropy of the water, including the sign, once all the water has come into thermal equilibrium.iii. Calculate the change in entropy of the fridge from part ii, including the sign.iv. Demonstrate that the Second Law has been obeyed in the process described in ii and iii.arrow_forwardThe pV diagram in the figure (Figure 1) shows a process abc involving 0.350 mol of an ideal gas. What was the temperature of this gas at points a, b, and c? How much heat had to be put in during the process to increase the internal energy of the gas by 1.30×104 J ?arrow_forwardIn the pV diagram shown in the figure (Figure 1), 80.0 J of work was done by 0.0610 mole of ideal gas during an adiabatic process. a) How much heat entered or left this gas from a to b? Express your answer in joules. b) By how many joules did the internal energy of the gas change? Express your answer in joules. c) What is the temperature of the gas at b? Express your answer in kelvins.arrow_forward
- 13 moles of monatomic ideal gas undergoes a thermodynamic process from point a to point c, as shown by the PV diagram in Figure 1. i) Determine the work done ON the gas from point a to point c. ii) Find the temperature of the gas at point b.arrow_forwardA gas follows the PV diagram in the figure below. Find the work done on the gas along the paths AB, BC, CD, DA, and ABCDA. (Enter your answers in J.) it wants it in the form i got wrong but i think my calculations are wrong for the only one i tried to doarrow_forwardAnswer the following questions about the PV diagram shown below. Please refer to the picture. Question A: How much work is performed in the process AB? Please give the answer in joules Question B: How much work is performed in the process BD? Please give the answer in joules Question C: How much work is peformed in the process AC? Please give the answer in joules Question D: How much work is performed in the process AD? Please give the answer in joules Question E: How much work is done by the gas in each cycle ADCA? Please give the answer in joules Question F: How much work is done by the gas in each cycle ADBA? Please give the answer in joulesarrow_forward
- Answer the following questions about the PV diagram shown below. Please refer to the picture. Question A: How much work is performed in the process AB? Please give the answer in joules Question B: How much work is performed in the process BD? Please give the answer in joules Question C: How much work is peformed in the process AC? Please give the answer in joulesarrow_forwardA volume of air (assumed to be an ideal gas) is first cooled without changing its volume and then expanded without changing its pressure, as shown by the path abc in the figure (Figure 1). Take the graduation po = 3.0 x 10°Pa and the graduation V = 0.06. Correct Part B How much heat does the air exchange with its surroundings during the process abc? Express your answer in joules using two significant figures. Figure Hνα ΑΣφ Р (Рa) Qabe = 4000 J a Submit Previous Answers Request Answer PoF b' X Incorrect; Try Again; One attempt remaining V (m³) Vo Part Carrow_forward2 moles of an ideal diatomic gas undergoes the three process cycle as depicted in the figure below. The cycle is composed of an isobaric, isovolumetric, and isothermal process. P. P. V, V. For the given parameters p.=P6=1.4 10° Pa and Va=0.7 m, V, = V.=0.35 m calculate the following. (Take the universal gas constant as R=8.314 J.mol1.K-1) a) The pressure p. = Pa b) The net work done by the gas W, J %3D net c) The heat entering the cycle QH= d) The thermal efficiency of the cycle (in percent): e = %arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY