
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 3, Problem 29EQ
Interpretation Introduction
Interpretation:
The products of the given reaction need to be identified.
Concept Introduction:
The formation of a carbon-carbon sigma bond occurs in the reaction of a Grignard reagent with ethylene oxide.
The Grignard reagent is usually alkyl magnesium bromide and written as an ion pair consisting of the MgBr+ ion and the alkyl anion. Below is the structure of Grignard reagent.
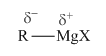
Here, alkyl anion acts as the nucleophile. This helps in the ring opening and a new sigma bond formation as shown below,
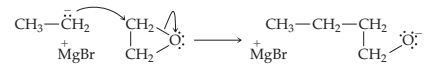
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
<
Question 28 of 85
Submit
1. LiN¹-Pr₂/THF
2. CH3CH2Br
CH3
A) This reaction would require a different
solvent.
B) The base is too weak to deprotonate this
compound.
C) There is a problem with the order of
addition.
D) The wrong nucleophilic position was used.
Tap here or pull up for additional resources
4.
A CHEM 245 student wants to synthesize some ethylene glycol to use as antifreeze in his
radiator this winter. He proposes the following reaction to his instructor, who quickly explains that this
reaction won't work as proposed due to the student's choice of reagent.
1) NaH
2) H20
OH
Но
a) Why can't sodium hydride (NaH) be used as the nucleophile in the reaction above?
b) Propose an alternate reagent that COULD be used with the ethylene oxide to successfully give the
desired ethylene glycol product.
Why is Ch3coo- a better nucleophile than OH-?
Chapter 3 Solutions
Pushing Electrons
Ch. 3 - Prob. 1EQCh. 3 - Prob. 2EQCh. 3 - Prob. 3EQCh. 3 - Prob. 4EQCh. 3 - Prob. 5EQCh. 3 - Prob. 6EQCh. 3 - Here are some exercises in sigma bond breaking....Ch. 3 - Prob. 8EQCh. 3 - Prob. 9EQCh. 3 - Prob. 10EQ
Ch. 3 - Prob. 11EQCh. 3 - Prob. 12EQCh. 3 - Prob. 13EQCh. 3 - Prob. 14EQCh. 3 - Prob. 15EQCh. 3 - Prob. 16EQCh. 3 - Prob. 17EQCh. 3 - Prob. 18EQCh. 3 - Prob. 19EQCh. 3 - Prob. 20EQCh. 3 - Prob. 21EQCh. 3 - Prob. 22EQCh. 3 - Prob. 23EQCh. 3 - Prob. 24EQCh. 3 - Prob. 25EQCh. 3 - Prob. 26EQCh. 3 - Prob. 27EQCh. 3 - Prob. 28EQCh. 3 - Prob. 29EQCh. 3 - Prob. 30EQCh. 3 - The reaction just described is reversible....Ch. 3 - Prob. 32EQCh. 3 - Prob. 59EQ
Knowledge Booster
Similar questions
- Draw structural formulas for organic products A and B in the window below. CH3 CH3CCH₂CI CH3 ● ● Mg ether // Draw only products having the organic portion of the original alkyl halide. Draw carbon-lithium bonds using the single bond tool. If a structure has a copper-lithium bond, do not draw the lithium. Separate products from different steps using the → sign from the drop-down menu. ? CH3OH A ChemDoodleⓇ B Sn [Farrow_forwardHow does this mechanism work if OH- is a nucleophile? Would the electrons from the bond be transferred to oxygen after the nucleophile attacks the electrophile? Or is it a simultaneous process?arrow_forwardO brandi.jamya Liked your me: HAPPY BIRTHDAY!! now This proposed alpha-alkylation reaction would not work. Identify what is wrong with it. 1. LiNi-Pr₂/THF 2. CH3CH2Br Tap here or pull up for additional resources A CH3 B This reaction would require a different solvent. The wrong base was used. There is a problem with the order of addition. Submit D The nucleophile isn't appropriate for this electrophile.arrow_forward
- A nucleophilic addition that starts with a terminal alkyne has __________ step(s), and yields a tertiary alcohol when the nucleophile attacks a(n) __________. 1, alcohol 3, formaldehyde 3, ketone 2, carboxylic acid 2, aldehydearrow_forwardDraw the organic product you would expect to isolate from the nucleophilic substitution reaction between the molecules shown. Note: You do not need to draw any of the side products of the reaction, only the substitution product. D xx + H₂O + X Click and drag to start drawing a structure.arrow_forwardWhat are the correct IUPAC names for ALL possible monohalogenation substrate products that could form when the substrate 2-methylbutane undergoes monohalogenation with Cl2 in the presence of UV light. a. 3-chloro-2-methylbutane b. 1-chloro-2-methylbutane c. 1,2-dichloro-2-methylbutane d. 2-chloro-2-methylbutane e. 4-chloro-2-methylbutane f. 2-chloro-3-methylbutane g.1-chloro-3-methylbutane h. 1,2-dichloro-3-methylbutanearrow_forward
- Draw the structure of the organic product formed when the given compounds undergo the three-step reaction sequence indicated. Select Draw Rings More Erase C Br 1. NaOC,H5. C,H5OH 2. NaOH, H2O 3. H3O*, heatarrow_forwardFor the given SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group. Include wedge-and-dash bonds and draw hydrogen on a stereocenter. HI.... + CI Draw the organic product. :C=N to H Organic product + Inorganic product Draw the inorganic product. ūarrow_forwardLook at the atom in each molecule and classify as an electrophile or a nucleophile. How do you know if it is a electrophile or nucleophile? What makes it an electro or nucleophile? C in CH3Br N in NH3 O in HO-arrow_forward
- Identify the role/function of the indicated molecule in the reaction? 1. LIAIH, ether 2. Но NaN3 Br -NH2 a. Nucleophile b. Electrophile c. Base O d. Acidarrow_forwardWhich of the following can behave as an electrophile? A. HBr B. Cl- C. CH3O- D. CH3CH2CH2CH3arrow_forward1. For each of the following molecules, put a box around the nucleophilic atom(s). MgBr S 点 H.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY