
Concept explainers
Interpretation:
The structure for a compound with molecular formula
Concept Introduction:
Determination of structure of a compound from its proton NMR spectrum:
There are several steps to be followed for the determination of the structure as given below.
Step
Calculation of hydrogen deficiency index (HDI) from the molecular formula.
It can be formulated as given below.
Where,
If
If
If
Step
Consider the number of signals and integration of each signal. This gives the clues about the symmetry of the compound.
In order to convert the integration values into useful information, a smallest number should be chosen and then all integration values should be divided by this number. If the numerical values are not whole numbers, then in order to arrive at whole numbers all the numbers should be multiplied by a suitable integer.
Step
Analyze each signal and then draw fragments consistent with each signal. Consider chemical sift value, integration and multiplicity. If
Step
Assemble the fragments into a molecular structure.
Important chemical shift values and splitting patterns:
An ethyl group is characterized by a triplet of
| Type of proton | Chemical shift |
| Methyl group | |
| Methylene group | |
| Methine group | |
Allylic  | |
Alkynyl  | |
Aromatic methyl 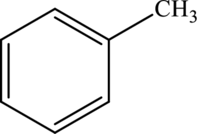 | |
Alkyl halide 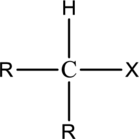 | |
Alcohol 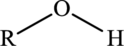 | |
Vinylic  | |
Aryl 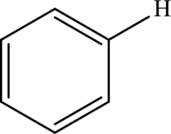 | |
Aldehyde 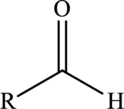 | |
Carboxylic acid 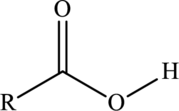 |
Key point:
Methyl protons, methylene protons and methine protons attached to a carbonyl group give a chemical shift value of
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
- The 1H NMR of a compound with molecular formula C5H7BrO is shown. Determine its structure.arrow_forwardA compound with molecular formula C5H10O2has the following NMR spectrum. Determine the number of protons giving rise to the signals at 2.0 ppm and 4.0 ppm.arrow_forwardPlease look at the Proton NMR spectrum and identify the proposed molecular structure. There is also a signal at 9.769 ppm indicating that an aldehyde is presentarrow_forward
- You are provided an unknown sample with the molecular formula C8H9NO. After running the NMR, you obtain the following spectra. Name and draw the structure of the compoundarrow_forwardDetermine the structure of the compound C6H100 whose 'H NMR spectrum is shown here. C6H100 6.5 25 7 6 3 1 Chemical shift (ppm)arrow_forwardThe following NMR spectra were obtained from a compound with the molecular formula C4H6O. Use this information to predict its structure.arrow_forward
- Below are the ¹H NMR spectrum of triphenylmethanol, benzophenone, and bromobenzene. Identify the compound corresponding to each ¹H NMR spectrum and draw the structure next to the ¹H NMR spectrum. Assign ALL peaks in each of the three ¹H NMR spectra. Hint: Conjugated systems (benzophenone) including an electronegative atom will cause a more downfield shift of ring protons in ¹H NMR compared with non-conjugated systems (bromobenzene). 8 8 8 7 7 7 6 6 6 5 5 5 4 PPM 4 PPM 4 PPM 3 3 3 2 2 2 1 1 1 0 0 0arrow_forwardThe 1H-NMR spectrum of an unknown compound shows two signals, each of which is a singlet. Explaining your choice, predict which of compounds A-D could the unknown could be?arrow_forwardThe1H NMR spectrum of CH3OH recorded on a 500 MHz NMR spectrometer consists of two signals, one due to the CH3 protons at 1715 Hz and one due to the OH proton at 1830 Hz, both measured downfield from TMS. (a) Calculate the chemical shift of each absorption. (b) Do the CH3 protons absorb upfield or downfield from the OH proton?arrow_forward
- The following is a proton NMR spectrum of a an alkyl halide with the formula C3H6Br What is the name of the compound?arrow_forwardYou complete the following problems by drawing the structure of the compound associated with the proton NMR spectra providedarrow_forwardHow could you distinguish the 1H NMR spectra of the following compounds?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





