
The chapter sections to review are shown in parentheses at the end of each problem.
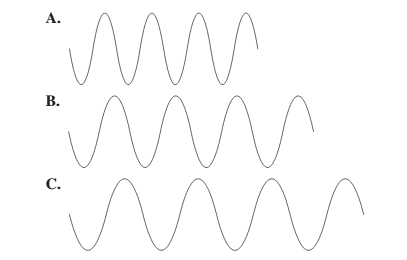
Use the following diagram tor problems 5.77 and 5.78:
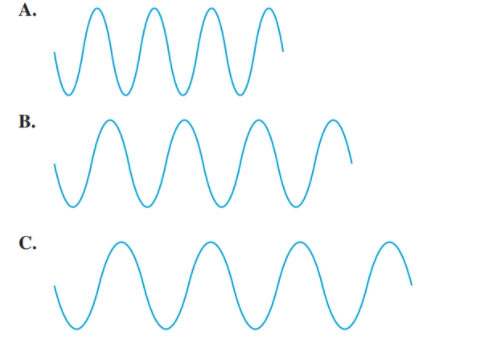
Select diagram A, B, or C that (5.1)
a. has the longest wavelength
b. has the shortest wavelength
c. has the highest frequency
d. has the lowest frequency
(a)
Interpretation:
The diagram having the longest wavelength should be selected.
Concept Introduction:The distance between two successive identical parts of the wave (from successive trough to trough or crest to crest) is said to be the wavelength.
Answer to Problem 77UTC
C.
Explanation of Solution
Given:
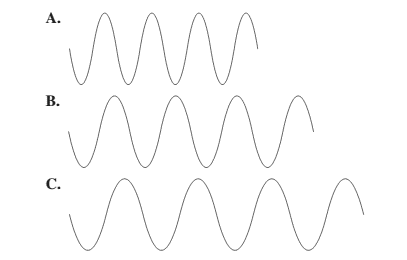
Larger the distance between the successive crest to crest or trough to trough, larger is the wavelength.
Among the given waves, the distance between successive crest to crest of wave C is largest so, the wavelength of wave C is longest.
(b)
Interpretation:
The diagram having the shortest wavelength should be selected.
Concept Introduction: The distance between two successive identical parts of the wave (from successive trough to trough or crest to crest) is said to be the wavelength.
Answer to Problem 77UTC
A.
Explanation of Solution
Given:
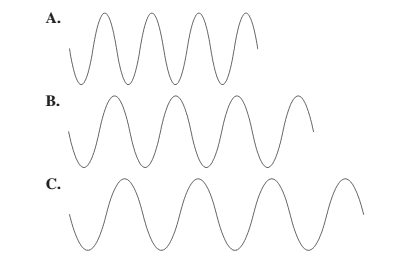
Smaller the distance between the successive crest to crest or trough to trough, smaller is the wavelength.
Among the given waves, the distance between successive crest to crest of wave A is smallest so, the wavelength of wave A is shortest.
(c)
Interpretation:
The diagram having the highest frequency should be selected.
Concept Introduction: The relationship between wavelength and frequency is given as:
Where
Answer to Problem 77UTC
A.
Explanation of Solution
Given:
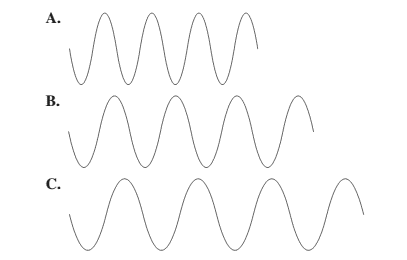
There is a relationship between wavelength and frequency that is they are inversely proportional to each other. Higher the value of wavelength, lower will be the frequency and vice-versa.
Thus, wave A will have highest frequency.
(d)
Interpretation:
The diagram having the lowest frequency should be selected.
Concept Introduction: The relationship between wavelength and frequency is given as:
Where
Answer to Problem 77UTC
C.
Explanation of Solution
Given:
There is a relationship between wavelength and frequency that is they are inversely proportional to each other. Lower the value of wavelength, higher will be the frequency and vice-versa.
Thus, wave C will have lowest frequency.
Want to see more full solutions like this?
Chapter 5 Solutions
Basic Chemistry
- Name the element that corresponds to each of the following:(5.4, 5.5, 5.6)a. 1s22s22p63s23p64s13d5b. [Xe]6s24f 145d106p5c. halogen with the highest ionization energy d. Group 2A (2) element with the lowest ionization energye. Period 4 element with the smallest atomic sizearrow_forwardConsider three elements with the following abbreviatedelectron configurations: (5.4, 5.5, 5.6)X = [Ar]4s23d5 Y = [Ar]4s23d104p1 Z = [Ar]4s23d104p6a. Identify each element as a metal, nonmetal, or metalloid.b. Which element has the smallest atomic size?c. Which element has the highest ionization energy?d. Which element has a half-filled sublevel?arrow_forward4. If you were given an antique piece of jewelry and you were told that it was made of solid gold, how could you devise a test to indicate that it was actually made of gold? 6,7.5arrow_forward
- * Calculate the energy electron from of for the transition of the h=8 to the n-I leve a hydrogen atom ΔΕΞ Joules Is this absorption or an emission process? K $21.0 ( 81.29 )( unarrow_forward12. Determine the number of electrons present in O (oxygen ion) when gO6 has received two electrons. The answer will be? (1.0 point)arrow_forward(5) Which is the larger species in each pair? a. Li or Lit b. I or Cst C. Cr or Cr3+ d. O or O24 I IDOS)arrow_forward
- 6.5.1 What is the minimum uncertainty in the position of anelectron moving at a speed of 4 × 106 m/s ± 1 percent?(The mass of an electron is 9.11 × 10−31 kg.)a) 2 × 10−8m d) 7 × 10−8m b) 1 × 10−9m e) 1 × 10−12 mc) 6 × 10−9marrow_forward(8.2)Place the following types of electromagnetic radiation in order of increasing wavelength. ultraviolet light, gamma rays, radio waves O ultraviolet light < gamma rays < radio waves O radio waves < ultraviolet light < gamma rays O gamma rays < radio waves < ultraviolet light O gamma rays ultraviolet light < radio waves O radio waves < gamma rays < ultraviolet lightarrow_forwardThe value of V (x, y, z) for an electron in an atom is equal to the energy of the electron at the point (x. y, 2) O the de Broglie wavelength of the electron o the probability of finding the electron near the point (x. y. 2) O none of the abovearrow_forward
- The most prevalent isotope of gold is Au-197. (4.5)a. How many protons, neutrons, and electrons are in this isotope?b. What is the atomic symbol of another isotope of gold with 116 neutrons?c. What is the atomic symbol of an atom with an atomic number of 78 and 116 neutrons?arrow_forwardWhat are the predicted values of JP for ¹0 and ¹30?arrow_forward11:11 ÔN A D Instructions - Classes - C... https://coetbschemfabon.neolm. Fill in the blanks: (4.1) Write the complete atomic/nuclide symbol for (a) an ion fluoride that contains 9 protons and 10 neutrons in the nucleus and 10 extranuclear electrons? (b) an ion of iron that contains 26 protons and 30 neutrons in the nucleus and 24 extranuclear electrons? Note: Use the "^" caret symbol to denote exponential components..for Ca¯“can be written as example, 20 "20^40Ca^-2" (atomic number written first). Answer: (a) mass number nuclide symbol (b) mass number nuclide symbol Continue > Status Dausearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





