
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6CTQ
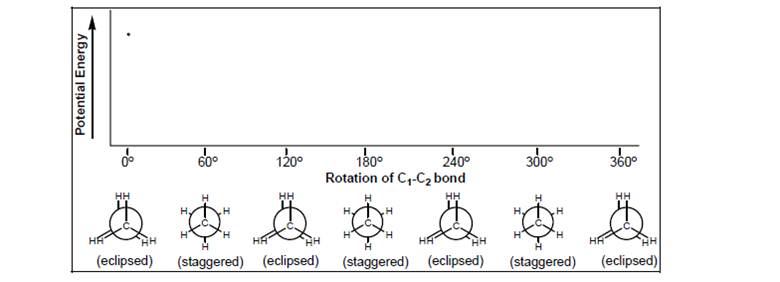
Complete this graph of relative potential energy vs. rotation of the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Why is the bond-line drawing of the allylic cation shown below insufficient?
O
(+)
The two electrons in the pi bond are delocalized into the adjacent p-orbital and are thus associated with three atom
spread out over two locations. Neither of these attributes is depicted by this bond-line drawing.
Carbon only has six electrons in its valence shell, and that is not allowed.
Bond-line drawings need to have hydrogen atoms drawn in.
There are four delocalized electrons spread over three atoms, which is not depicted by this bond-line drawing.
1. Make a qualitative plot of 4-ethyl-2-methylhexane showing the energy changes that arise from a 360°
rotation about the C3-C4 bond. You do not need the actual numerical values of the energy changes, but you
should label all maxima and minima with the appropriate Newman projections.
Determine α' and α for a molecule that experiences a Debye bond energy of -2 aJ
being at a distance of 0.1 nm from a molecule of acetic acid
Chapter 6 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 6 - Prob. 1CTQCh. 6 - Prob. 2CTQCh. 6 - Prob. 4CTQCh. 6 - Prob. 5CTQCh. 6 - Complete this graph of relative potential energy...Ch. 6 - Prob. 7CTQCh. 6 - Prob. 8CTQCh. 6 - Prob. 9CTQCh. 6 - Consider the Newman projection below. a. Draw a...Ch. 6 - Draw a Newman projection showing the lowest P.E....
Ch. 6 - Prob. 12CTQCh. 6 - Prob. 13CTQCh. 6 - In skeletal representations the hydrogens are not...Ch. 6 - Prob. 15CTQCh. 6 - Prob. 16CTQCh. 6 - Prob. 17CTQCh. 6 - Prob. 19CTQCh. 6 - Prob. 20CTQCh. 6 - Prob. 21CTQCh. 6 - Prob. 22CTQCh. 6 - Prob. 23CTQCh. 6 - Draw a constitutional isomer of pentane,...Ch. 6 - How many H’s are lost from the molecular formula...Ch. 6 - How many ifs are lost from the molecular formula...Ch. 6 - Prob. 27CTQCh. 6 - What is the degree of unsaturation for the example...Ch. 6 - Without counting hydrogens, determine which one of...Ch. 6 - Determine the degree of unsaturation (and draw a...Ch. 6 - a model of each molecule shown above: Is the...Ch. 6 - Prob. 32CTQCh. 6 - Prob. 33CTQCh. 6 - Label each double bond E, Z, or neither. (It may...Ch. 6 - Prob. 35CTQCh. 6 - Prob. 36CTQCh. 6 - Indicate the relationship between each pair....Ch. 6 - Prob. 38CTQCh. 6 - Prob. 1ECh. 6 - Prob. 2ECh. 6 - Using your model of butane (CH3CH2CH2CH3) ,...Ch. 6 - Consider the molecule 1-bromo-2-methylbutane. C3...Ch. 6 - Prob. 5ECh. 6 - Prob. 8ECh. 6 - Prob. 9ECh. 6 - Prob. 10ECh. 6 - Prob. 11ECh. 6 - Prob. 12ECh. 6 - Prob. 13ECh. 6 - Prob. 15ECh. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - Double bonds do not rotate freely under normal...Ch. 6 - up an example (not appearing in this ChemActivity)...Ch. 6 - Prob. 24ECh. 6 - Prob. 25E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ОН HO "ОН Н ОН OH O,. ОН NH2 This is the molecule commonly known as Doxorubicin. Doxorubicin is used in chemotherapy to inhibit uncontrolled cell growth, a hallmark of cancerous cells. Doxorubicin specifically inhibits an enzyme called topoisomerase which helps remove supercoils in DNA as DNA is replicated during cell growth and division (mitosis). the following key to label the different Indicate the functional groups a part of Doxorubicin usi parts of the molecule (note not all functional groups listed may be a part of Doxorubicin): Carboxylic Acid label with a C Ketone label with a K Alcohol label with an A Ketal label with a T Aldehyde label with an H Ester label with an R Acetal label with an L Amine label with an M Amide label with a D Phenol label with a P Aromatic label with a B Ether label with an Earrow_forwardAn electrostatic potential map of calicene is shown below. a) Both the electrostatic potential map and its significant dipole moment indicate that calicene is an unusually polar hydrocarbon. Which of the dipolar resonance forms, structure A or structure B, better corresponds to the electron distribution in the molecule? Select the single best answer. b) Which one of the following structures should be stabilized by resonance to a greater extent? Select the single best answer.arrow_forwardDraw the molecular orbital diagram of a linear C-H (i.e. one carbon atom bonded to one hydrogen atom). Draw out the MOs for orbitals containing electrons. 1. Would you expect C-H to behave as a carbocation, carbanion, or radical? 2. Would removal of one electron from C-H to generate C-H+ increase or decrease the bond length between carbon and hydrogen?arrow_forward
- 4. Explain why the protons on the sp³-hybridized carbon of propene (CH3-CH=CH₂) are more acidic than the protons on the sp²-hybridized carbons of propene, even though sp2-hybridized carbons are more electronegative than sp³-hybridized carbons. Use both structures and sentences in your answer.arrow_forward5. Chairs and E2 b. a. Draw the line-bond structure of (1R, 2S,3S)-1-ethyl-2-iodo-3-isopropylcyclohexane. Draw both chair flips of (1R, 2S,3S)-1-ethyl-2-iodo-3-isopropylcyclohexane. Showing all calculations, determine which is the more stable conformation. C. Which chair flip is able to undergo E2 elimination? Justify your answer. d. Show the product of such an E2 elimination using NaOEt as base.arrow_forwardChapter 2 [References] H3C HB CH3 На, CH3 CH3 D H3C B НО Cholestanol differs from cholesterol only in the absence of a double bond in ring B. Draw the three-dimensional structure of cholestanol, and then determine the orientation of the following groups: #1: H at the junction of rings C & D with respect to ring C| #2: H at the junction of rings A & B with respect to ring B #3: Methyl at the junction of rings C & D with respect to ring C| O Iarrow_forward
- What is the order of the punch and the energy diagram according to the T.O.M molecular theory of F2?arrow_forwardThe figure below represents the highest accupied molecular orbitals (HOMOS) and the lowest unoccupied molecular orbital (LUMO) for an organic compound with a ketone functional group. The and n orbitals represent the HOMOS and the a* represents the LUMO orbital. л" E I Nonpolar solvent I* Л Polar solvent To The left side HOMOS and LUMO represent the molecule in a nonpolar solvent, and the right side represent the HOMOS and LUMD in a polar solvent, such as water. You can see the change in the energy of the HOMOS and LUMO when the polar solvent interacts with the electrons in the ann orbitals. Study the figure and choose which of the following statements are true or false about why there is a blue shift in the electronic transition. Select all that are True. the energy gap between the n MO and the n* MO is larger in the polar solvent than in the nonpolar solvent, so the photon needed to excite the electron would require more energy when the molecule is in the polar solvent the energy gap…arrow_forward3. For each of the following pairs of compounds, explain which has the larger dipole moment. Designate the direction of bond dipoles except for CC- and CH-bonds. Indicate the direction and estimated size (large, small, zero) of the overall net molecular dipole moment. For b) also indicate, which compound has the higher boiling point. a) b) c) Ô SO₂ CI H H CI CH3 Br C-Br CH3 CO₂ CI H Br H3C-C-Br CH3 & & CH3 CH3 CI Harrow_forward
- With reference to the indicated C-H bonds in the following compound, rank the C-H bonds in order of increasing bond strength. Be sure to answer all parts. H |2 H. 4 H H3 2. 1.arrow_forwardWhy does acetone [(CH3)2C=O, dipole moment = 2.69 D] have a larger dipole moment than phosgene [Cl₂C=O, dipole moment = 1.17 D] ? Note: electronegativities C = 2.5, Cl = 3.2, O= 3.5, H = 2.2 Select one: O A. In phosgene, the resultant of the C-Cl bond dipole moments is opposite to and partially cancels the C=O dipole moment. In acetone, the resultant of the C-H bond dipole moments (although small) adds to the C=O dipole moment. O B. Phosgene has a tetrahedral geometry while acetone possesses a trigonal geometry. O C. The C-H bond dipole moments of acetone are greater in magnitude than C-Cl bond dipole moments of phosgene. O D. In phosgene the C-Cl bond dipoles cancel out each other.arrow_forwarde) Describing the boding in ethene using VBT: One C=C bond, consisting of (state how many, 1,2,3 etc) (o or n) C-C bond/s formed from overlap of a orbital with a orbital and (state how many, 1,2,3 etc) (o or n) C-C bond/s formed from overlap of a orbital with a orbital. C-H bonds, (o or n) bonds formed from overlap of witharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY