
Concept explainers
Interpretation:
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which are determine the characteristic reactions taking place in it.
Alcohol: It is an organic compound where it contains at least one
Ether: Ether is a group of organic compound where two aryl or alkyl groups are connected by an oxygen atom. It is represented as
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
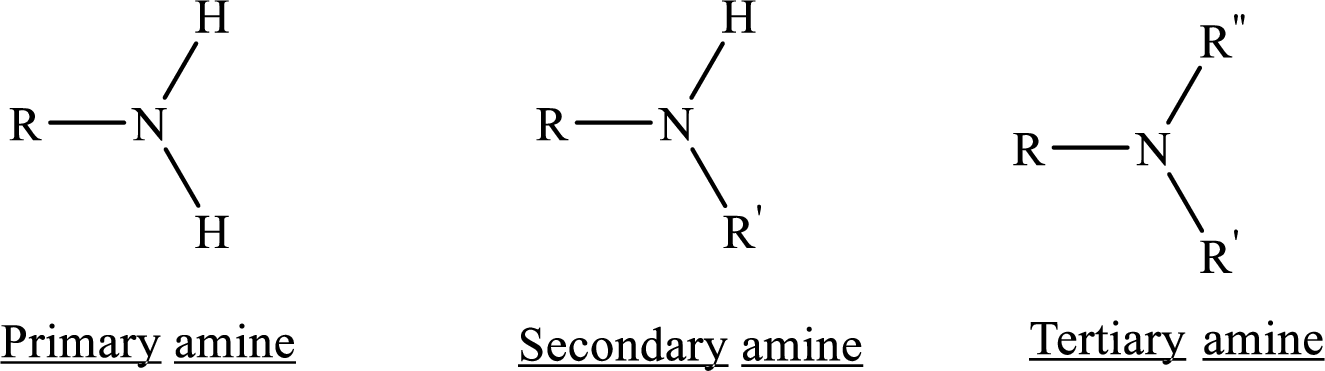
Ester: One
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry In Context
- III. Essay. Answer the following questions concisely. 1. Ether and alcohol are functional isomers. Give the functional isomer of 3- Methoxyhexane. Name and draw the condensed structural formula of this alcohol. 2. Both dimethyl ether and ethanol has the same molecular mass because they are functional isomers. Why is dimethyl ether a gas at room temperature while ethanol is a liquid? 3. Why has use of MTBE as a gasoline additive been discontinued in the United States?arrow_forward2. Some students confuse the ester and ether functional groups. Both linkages are formed by condensation reactions. a) Complete this statement: An ether linkage is formed by the condensation of: b) Use structures to show the condensation of cyclohexanol molecules to create the alternate synthesis product discussed in the report sheet of your Dehydration experiment (Lab 04).arrow_forwardCircle all the functional groups in each of these compounds.arrow_forward
- An alcohol or an alkane? Why? An ether or an aldehyde? Why? A ketone or a carboxylic acid? Why? Would propanoic acid (the carboxylic acid example in Table 1) be soluble in water? ____ Explain how you know this. Why does solubility decrease with increasing carbon chain length? Which functional group that we have studied so far has the highest boiling point? Explainarrow_forwardExplain Dr. Flor’s theory of “the intrinsic reactivity of all functional groups isconstant, independent of the molecular size”.arrow_forwardDescribe the functional group similarities and differences among the four molecules shown below. Compare their polarities; explain your answer. H. H. H. H. H. H. H. HIIarrow_forward
- Match the following organic compounds with their functional group.arrow_forwardBONUS. What is a functional group? Explain why it is helpful for organic chemists to classify molecules according to their functional groups?arrow_forward"Cyclohexene is a linear molecule with 6 carbon Describe the molecule cyclohexene and provide the chemical formula. atoms and 12 hydrogen atoms. There is a triple bond because it ends in - # 4 (Glycerina) ene. The chemical formula is C6H12" Which two functional groups are in the molecule shown below? Use the terms left and right to distinguish them. "The left functional group is a carboxylic acid. The right functional group is an #5 (Trinitress) alcohol." Harrow_forward
- Give examples of two different functional groups AND what property they give to the molecule they are found on.arrow_forward6. What type of organic molecule is this? он ketone alcohol aldehyde organic acidarrow_forwardHow does the structure of an alcohol differ from an ether? Describe how an aldehyde differs in structure from a ketone. Thiols are compounds which resemble alcohols, except that the oxygen atom is replaced by a sulfur atom. Draw the analogous thiol for the four carbon alcohol in Table 1. Describe the structural difference between carboxylic acids and esters. Are ethers polar molecules? Would you expect ethers to have higher or lower boiling points than alkanes (circle one)? Explain. Pentane (an alkane) has a boiling point of 36 °C. Does the data agree with your prediction? explain why this could be the casearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



