
Chemistry For Changing Times (14th Edition)
14th Edition
ISBN: 9780321972026
Author: John W. Hill, Terry W. McCreary
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 11RQ
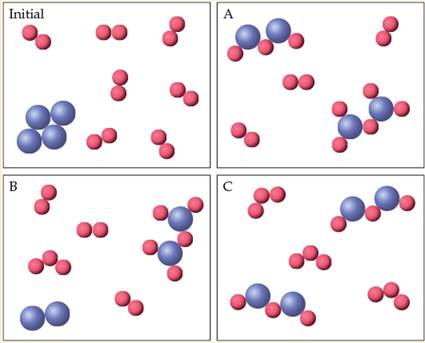
In the figure. the blue spheres represent phosphorus atoms, and the red ones represent oxygen atoms. The box labeled “lnitial represents a mixture. Which one of the other three boxes (A, B. or C) could not represent that mixture after a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Chemistry For Changing Times (14th Edition)
Ch. 2 - Prob. 1RQCh. 2 - Prob. 2RQCh. 2 - Prob. 3RQCh. 2 - Prob. 4RQCh. 2 - Cavendish found that water was composed of two...Ch. 2 - Prob. 6RQCh. 2 - Fructose (fruit sugar) is always composed of 40,0%...Ch. 2 - Outline the main points of Dalton's atomic theory,...Ch. 2 - Prob. 9RQCh. 2 - Prob. 10RQ
Ch. 2 - In the figure. the blue spheres represent...Ch. 2 - 12. a. How is Avogadro’s number linked with the...Ch. 2 - Prob. 13RQCh. 2 - Prob. 14RQCh. 2 - Prob. 15PCh. 2 - 16. An iron nail dissolves in a solution of...Ch. 2 - If you place a 400 g effervescent antacid pill...Ch. 2 - Prob. 18PCh. 2 - 19, Acetylene, used for welding, contains 24.02 g...Ch. 2 - 20. Nitrous oxide (N2O, "laughing gas") contains...Ch. 2 - Prob. 21PCh. 2 - Prob. 22PCh. 2 - Prob. 23PCh. 2 - Prob. 24PCh. 2 - When 18.029 of water is decomposed by...Ch. 2 - Prob. 26PCh. 2 - Prob. 27PCh. 2 - Prob. 28PCh. 2 - Prob. 29PCh. 2 - Prob. 30PCh. 2 - 31. Use Dalton's atomic theory to explain what is...Ch. 2 - Prob. 32PCh. 2 - Hydrogen and oxygen combine in a mass ratio of...Ch. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - A compound containing only oxygen and rubidium has...Ch. 2 - 37. A sample of an oxide of tin with the formula...Ch. 2 - 38. Consider three oxides of nitrogen, X, Y, and...Ch. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - A blue solid called azulene is thought to be a...Ch. 2 - Prob. 46PCh. 2 - Prob. 47APCh. 2 - Prob. 48APCh. 2 - Prob. 49APCh. 2 - Prob. 50APCh. 2 - 51. See Table 2.1 . Another compound of nitrogen...Ch. 2 - Prob. 52APCh. 2 - Prob. 53APCh. 2 - Prob. 54APCh. 2 - Prob. 55APCh. 2 - Prob. 56APCh. 2 - Prob. 57APCh. 2 - Prob. 58APCh. 2 - Prob. 2.1CTECh. 2 - When water is electrolyzed, from each one molecule...Ch. 2 - A health-food store has a large display of...Ch. 2 - Prob. 2.4CTECh. 2 - Prob. 2.5CTECh. 2 - Prob. 2.6CTECh. 2 - Prob. 1CGPCh. 2 - Prob. 2CGPCh. 2 - Prob. 3CGPCh. 2 - Prob. 4CGPCh. 2 - Prob. 5CGPCh. 2 - Materials Needed: Alka-Seltzer tablets (8) 1/4 cup...Ch. 2 - Materials Needed: Alka-Seltzer tablets (8) 1/4 cup...Ch. 2 - Prob. 3CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Copper atoms. (a) What is the average mass of one copper atom? (b) Students in a college computer science class once sued the college because they were asked to calculate the cost of one atom and could not do it. But you are in a chemistry course, and you can do this. (See E. Felsenthal, Wall Street Journal, May 9, 1995.) If the cost of 2.0-mm diameter copper wire (99.9995% pure] is currently 41.70 for 7.0 g, what is the cost of one copper atom?arrow_forwardThe early alchemists used to do an experiment in which water was boiled for several days in a sealed glass container. Eventually. some solid residue would appear in die bottom of the flask, which was interpreted to mean that some of the water in the flask had been converted into earth. When Lavoisier repeated this experiment, he found that the water weighed the same before and after heating, and the mass of die flask plus the solid residue equaled the original mass of the flask. Were the alchemists correct? Explain what really happened. (This experiment is described in the article by A. F. Scott in Scientific American, January 1984.)arrow_forwardReference Section 5-2 to find the atomic masses of 12C and 13C, the relative abundance of 12C and 13C in natural carbon, and the average mass (in u) of a carbon atom. If you had a sample of natural carbon containing exactly 10,000 atoms, determine the number of 12C and 13C atoms present. What would be the average mass (in u) and the total mass (in u) of the carbon atoms in this 10,000-atom sample? If you had a sample of natural carbon containing 6.0221 1023 atoms, determine the number of 12C and 13C atoms present What would be the average mass (in u) and the total mass (in u) of this 6.0221 1023 atom sample? Given that 1 g = 6.0221 1023 u, what is the total mass of I mole of natural carbon in units of grams?arrow_forward
- In 1886 Eugene Goldstein observed positively charged particles moving in the opposite direction to electrons in a cathode ray tube (illustrated below). From their mass, he concluded that these particles were formed from residual gas in the tube. For example, if the cathode ray tube contained helium, the canal rays consisted of He+ ions. Describe a process that could lead to these ions. Canal rays. In 1886, Eugene Goldstein detected a stream of particles traveling in the direction opposite to that of the negatively charged cathode rays (electrons). He called this stream of positive particles "canal rays:"arrow_forwardThere are 1.699 1022 atoms in 1.000 g of chlorine. Assume that chlorine atoms are spheres of radius 0.99 and that they are lined up side by side in a 0.5-g sample. How many miles in length is the line of chlorine atoms in the sample?arrow_forwardThese questions concern the work of J. J. Thomson: From Thomson’s work, which particles do you think he would feel are most important in the formation of compounds (chemical changes) and why? Of the remaining two subatomic particles, which do you place second in importance for forming compounds and why? Come up with three models that explain Thomson’s findings and evaluate them. To be complete you should include Thomson’s findings.arrow_forward
- Early tables of atomic weights (masses) were generated by measuring the mass of a substance that reacts with 1.00 g of oxygen. Given the following data and taking the atomic mass of hydrogen as 1.00, generate a table of relative atomic masses for oxygen, sodium, and magnesium. Element Mass That Combines with 1.00g Oxygen Assumed Formula Hydrogen 0.126 g HO Sodium 2.875 g NaO Magnesium 1.500 g MgO How do your values compare with those in the periodic table? How do you account for any differences?arrow_forwardAn adult human body contains 6.0 L blood, which contains about 15.5 g hemoglobin per 100.0 mL blood. The molar mass of hemoglobin is approximately 64,500 g/mol and there is 4 mol iron per 1 mol hemoglobin. A news item claims that there is sufficient iron in the hemoglobin of the body that this iron, if it were in the form of metallic iron, could make a 3-in. iron nail that weighs approximately 3.7 g. Show sufficient calculations to either support or refute the claim.arrow_forwardAn element X bas five major isotopes, which are listed below along with their abundances. What is the element? Isotope Percent Natural Abundance Mass (u) 46x 8.00% 45.95232 47x 7.30% 46.951764 48x 73.80% 47.947947 49x 5.50% 48.947841 50x 5.40% 49.944792arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY