
Chemistry For Changing Times (14th Edition)
14th Edition
ISBN: 9780321972026
Author: John W. Hill, Terry W. McCreary
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 2, Problem 53AP
Interpretation Introduction
Interpretation:
Law should be identified for the production of carbon dioxide gas from HCl reacted with sodium carbonate.
Concept introduction:
Law of conservation of mass explains the the mass of product remain equivalent to mass of reactant as number of moles produced get balanced with mole reacted as follows:
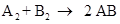
Thus, 1 mol of molecule A reacts with 1 mole of molecule B to produce 2 mole of compound AB and therefore the mole produced is equivalent to mole reacted.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Chemistry For Changing Times (14th Edition)
Ch. 2 - Prob. 1RQCh. 2 - Prob. 2RQCh. 2 - Prob. 3RQCh. 2 - Prob. 4RQCh. 2 - Cavendish found that water was composed of two...Ch. 2 - Prob. 6RQCh. 2 - Fructose (fruit sugar) is always composed of 40,0%...Ch. 2 - Outline the main points of Dalton's atomic theory,...Ch. 2 - Prob. 9RQCh. 2 - Prob. 10RQ
Ch. 2 - In the figure. the blue spheres represent...Ch. 2 - 12. a. How is Avogadro’s number linked with the...Ch. 2 - Prob. 13RQCh. 2 - Prob. 14RQCh. 2 - Prob. 15PCh. 2 - 16. An iron nail dissolves in a solution of...Ch. 2 - If you place a 400 g effervescent antacid pill...Ch. 2 - Prob. 18PCh. 2 - 19, Acetylene, used for welding, contains 24.02 g...Ch. 2 - 20. Nitrous oxide (N2O, "laughing gas") contains...Ch. 2 - Prob. 21PCh. 2 - Prob. 22PCh. 2 - Prob. 23PCh. 2 - Prob. 24PCh. 2 - When 18.029 of water is decomposed by...Ch. 2 - Prob. 26PCh. 2 - Prob. 27PCh. 2 - Prob. 28PCh. 2 - Prob. 29PCh. 2 - Prob. 30PCh. 2 - 31. Use Dalton's atomic theory to explain what is...Ch. 2 - Prob. 32PCh. 2 - Hydrogen and oxygen combine in a mass ratio of...Ch. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - A compound containing only oxygen and rubidium has...Ch. 2 - 37. A sample of an oxide of tin with the formula...Ch. 2 - 38. Consider three oxides of nitrogen, X, Y, and...Ch. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - A blue solid called azulene is thought to be a...Ch. 2 - Prob. 46PCh. 2 - Prob. 47APCh. 2 - Prob. 48APCh. 2 - Prob. 49APCh. 2 - Prob. 50APCh. 2 - 51. See Table 2.1 . Another compound of nitrogen...Ch. 2 - Prob. 52APCh. 2 - Prob. 53APCh. 2 - Prob. 54APCh. 2 - Prob. 55APCh. 2 - Prob. 56APCh. 2 - Prob. 57APCh. 2 - Prob. 58APCh. 2 - Prob. 2.1CTECh. 2 - When water is electrolyzed, from each one molecule...Ch. 2 - A health-food store has a large display of...Ch. 2 - Prob. 2.4CTECh. 2 - Prob. 2.5CTECh. 2 - Prob. 2.6CTECh. 2 - Prob. 1CGPCh. 2 - Prob. 2CGPCh. 2 - Prob. 3CGPCh. 2 - Prob. 4CGPCh. 2 - Prob. 5CGPCh. 2 - Materials Needed: Alka-Seltzer tablets (8) 1/4 cup...Ch. 2 - Materials Needed: Alka-Seltzer tablets (8) 1/4 cup...Ch. 2 - Prob. 3CHQ
Knowledge Booster
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY