
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 3.2P
(a) Classify the carbon atoms in each compound as
[1] 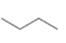 [2]
[2] [3]
[3]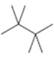 [4]
[4]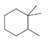
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider the tricyclic structure A. (a) Label each substituent on the rings as axial or equatorial. (b) Draw a skeletal structure
for A, using wedges and dashes to show whether the substituents are located above or below the rings.
Consider the tricyclic structure A. (a) Label each substituent on the rings as axial or equatorial. (b) Draw a skeletal structure for A, using wedges and dashed wedges to show whether the substituents are located above or below the rings.
Dexamethasone is a halogen-containing steroid used to treat infl ammation in rheumatoid arthritis and other conditions. (a) Classify the alkyl halide in dexamethasone as 1 °, 2 °, or 3 °. (b) Classify the hydroxyl groups as 1 °, 2 °, or 3 °.
Chapter 3 Solutions
Organic Chemistry
Ch. 3 - Prob. 3.1PCh. 3 - (a) Classify the carbon atoms in each compound as...Ch. 3 - Problem 3.3 Classify a carbon atom by the number...Ch. 3 - Classify each alkyl halide and alcohol as , or...Ch. 3 - Prob. 3.5PCh. 3 - Prob. 3.6PCh. 3 - Draw the structure of a compound of molecular...Ch. 3 - Prob. 3.8PCh. 3 - Prob. 3.9PCh. 3 - Draw the structure of a compound fitting each...
Ch. 3 - Draw structures that fit each description and name...Ch. 3 - What types of intermolecular forces are present in...Ch. 3 - Which compound in each pair has the higher boiling...Ch. 3 - Explain why the boiling point of propanamide, is...Ch. 3 - Predict which compound in each pair has the higher...Ch. 3 - Prob. 3.16PCh. 3 - Which compounds are water soluble? a. b. c.Ch. 3 - a Label the hydrophobic and hydrophilic portions...Ch. 3 - Prob. 3.19PCh. 3 - Prob. 3.20PCh. 3 - Prob. 3.21PCh. 3 - Prob. 3.22PCh. 3 - Problem 3.23 (a) What types of intermolecular...Ch. 3 - Prob. 3.24PCh. 3 - Prob. 3.25PCh. 3 - Problem 3.26 Label the electrophilic and...Ch. 3 - Problem 3.27 Considering only electron density,...Ch. 3 - Prob. 3.28PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 3.30PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 3.36PCh. 3 - Prob. 3.37PCh. 3 - Prob. 3.38PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 3.43PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 3.45PCh. 3 - 3.46 Rank the following compounds in order of...Ch. 3 - 3.47 Which of the following molecules can hydrogen...Ch. 3 - 3.48 Explain why diethylether and have similar...Ch. 3 - Prob. 3.49PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 3.51PCh. 3 - Prob. 3.52PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 3.54PCh. 3 - Prob. 3.55PCh. 3 - 3.56 Label the electrophilic and nucleophilic...Ch. 3 - 3.57 By using only electron density arguments,...Ch. 3 - 3.58 The composition of a cell membrane is not...Ch. 3 - Prob. 3.59PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 3.62PCh. 3 - Prob. 3.63PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 15) Give the IUPAC name for each compound.arrow_forward3. Consider the following structures: Br H3C- CH 3 C₂H5 -H -OH Structure A Br HO- H CH3 -CH3 -C₂H5 Structure B C₂H5 The relationship between structure A and B is: The relationship between structure A and C is: The relationship between structure A and D is: The relationship between structure B and C is: Br The relationship between structure B and D is: The relationship between structure C and D is: OH H CH3 CH3 (a) Designate each chirality center as (R) or (S) in structures A, B, C and D. (b) Fill in the blanks for the structural relationship between the compounds in each of the pairs that follow. They may be unrelated, identical (the same compound), constitutional isomers, diastereoisomers, enantiomers. Structure C H₂C H Br 4|||| C₂H5 ||||||CH 3 OH Structure Darrow_forwardexplain why methanethiol, CH3SH, has a lower boiling point (6°C) than methanol, CH3OH (65°C), even though methanethiol has a higher molecular weightarrow_forward
- 1) identify the reactant/s in the chemical equation and circle it, also name its major functional group 2) identify the product/s in the chemical equation (circle it) and name its functional group 3) is the a reversible reaction or not? How do we knowarrow_forwardOH H3C Type of Reaction: CH3 CH3 CH3 + OH Type of Reaction: + [0] НОarrow_forwardQuestion 1 Write the IUPAC name for each compound: сно CHO (a) (b) (e) Question 2 Write the IUPAC name for each compound: H,C CH, (a) CH,-CH(CH,)ẠCH, (b)arrow_forward
- Classify each pair of compounds as constitutional isomers or identical molecules.arrow_forward(ii) Name the following using IUPAC system of nomenclature: (a) CH2 (Cl) CH (NH2) CHO (b) CH3 C (Br) CH2 CH (F) COOH Brarrow_forwardClassify the following reaction as: addition, elimination, substitution or rearrangement. A) substitution CH3 B) addition CH3 OTS C) elimination D) rearrangementarrow_forward
- Name each compound. CH; CH, CH, · CH3-CH-CH-CH-CEC-H CH3arrow_forwardAssign (R) or (S) designations to each of the following compounds: (a) (b) HO (c) CH3 .CI H.arrow_forwardCompound A Compound F HCI CH3OH H₂SO4 Compound B CH3 CH₂ CH₂ Cl Compound E NaOH K₂Cr₂O7 H₂SO4 Compound C K₂Cr₂O7 H₂SO4 Compound D a. Draw the structural formulas of compounds A, C, D, E and F in the boxes provided above.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY