
Concept explainers
(a)
Interpretation:
The apparatus has to be redrawn if the temperature is increased from
Concept introduction:
Charles’s law: States that volume is directly proportional to temperature when the gas is held at constant pressure and number of molecules.
(a)
Answer to Problem 8.26UKC
According to Charles’s law, if temperature increases at constant pressure, then volume also increases. Therefore, the apparatus is redrawn as given below:
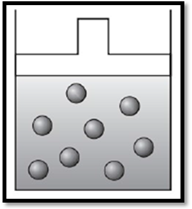
Explanation of Solution
Given data is that the temperature increases from
Charles’s law is the
From Charles’s law we know that
According to Charles’s law, if temperature increases at constant pressure, then volume also increases. Therefore, the apparatus is redrawn as given below:
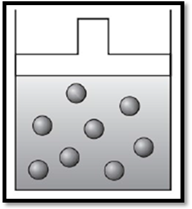
(b)
Interpretation:
The apparatus has to be redrawn if the pressure is increased from
Concept introduction:
Boyle’s law:
At fixed temperature, the volume of a fixed amount of gas is inversely proportional to the pressure exerted by the gas.
(b)
Answer to Problem 8.26UKC
According to Boyle’s law, if pressure increases at constant temperature, then volume decreases. Therefore, the apparatus is redrawn as given below:
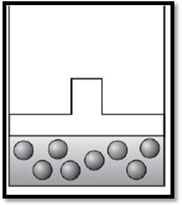
Explanation of Solution
Given data is that the pressure is increased from
Boyle’s law is the law which relates pressure and volume at a constant temperature and number of molecules.
From Boyle’s law:
According to Boyle’s law, if pressure increases at constant temperature, then volume decreases. Therefore, the apparatus is redrawn as given below:
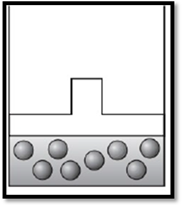
(c)
Interpretation:
The apparatus has to be redrawn if the temperature is decreased from
Concept introduction:
General Gas Law:
Combining Charles’s law and Boyle’s law we get the General gas law or combined gas law.
(c)
Answer to Problem 8.26UKC
The volume remains unchanged and the apparatus is redrawn as given below:
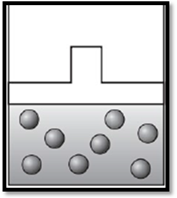
Explanation of Solution
Given data is that the temperature is decreased from
Combined gas law is proposed by combining the Boyle’s law and Charle’s law and is given by:
Therefore, the volume remains unchanged and the apparatus is redrawn as given below:
Want to see more full solutions like this?
Chapter 8 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- A 50-year-old man came to the emergency department after returning from foreign travel. His symptoms included persistent diarrhea (over the past 3 days) and rapid respiration (tachypnea). Blood gases were drawn with the following results: pH 7.21 pco2 19 mm Hg po2 96 mm Hg HCO3 − 7 mmol/L SO2 96% (calculated) (reference range, >95%) Question: What is the patient’s acid–base status? Why is the HCO3 − level so low? Why does the patient have rapid respiration?arrow_forwardA sample of 8.00 mol of gas in a 10.00 L container is at 45.0 °C. What is the pressure (in atm) of the gas?arrow_forwardIn 2011, researchers showed that hypochlorous acid (HClO) generated by white blood cells kills bacteria. Calculate the percent dissociation of (a) 0.40 M HClO; (b) 0.035 M HClO (Ka = 2.9x10-8).arrow_forward
- Heliox is a helium‑oxygen mixture that may be used in scuba tanks for divers working at great depths. It is also used medically as a breathing treatment. A 5.25 L tank holds helium gas at a pressure of 1734 psi. A second 5.25 L tank holds oxygen at a pressure of 461.0 psi. The two gases are mixed in a 5.25 L tank. If the temperature remains the same throughout the process, what is the pressure of the gas mixture in the tank? Assume ideal gas behavior.arrow_forwardConsider the following reaction at 25°C with the ΔG°’ = +1800 J/mol for the forward reaction.The molar concentrations at the beginning of the reaction were [A] = 19 mM and [B] = 10 mM.After 1 hour, the concentrations were [A] = 16 mM and [B] = 13 mM. Calculate the ΔG of the reaction at the 1 hour timepoint. Please round to 1 decimal point.Gas constant = 8.315 J/mol Karrow_forward(a) What is the specific volume of a gas at 1 250 kPaa and 33 C when its density is 0.715 kg/m3 at 101.325 kPaa and 0 C. [3 decimal places] (b) Calculate its gas constant and approximate molecular weight. [whole number]arrow_forward
- How many moles of chlorine gas at 120. °C and 33.3 atm would occupy a vessel of 39.0 L?arrow_forwardWhat mass of Co(s) may be deposited from an aqueous CoCl, solution if a current of 2.50 A is applied to the solution for 365 s? (e*rea(Co2*/Co) = -0.277 V, F = 96485 Cimol) 0.557 g 0.279 g O 1.01 g O 1.11 g 0.0772 garrow_forwardHow many liters of dry hydrogen gas, measured at 798 mmHg and 21C, will be released by the decomposition of 230 mL of H20 at 1.42atm and 30C? 2 H20 (g) → 2 H2 (g) + O2 (g) Answer to 3 decimal places. Your Answer: Answer unitsarrow_forward
- Ismail analyzed the triester content in an esterified oil using the gas chromatographic technique. The measurements for triester contents are 33.27, 33.37, and 33.34%. Calculate: (i) the average triester content (ii) the standard deviation, s for triester content (iii) the relative standard deviation for the triester contentarrow_forwardWhat volume in liters would 20.0 moles of sulfur dioxide occupy at 75.3 °C with a pressure of 2.40 atm?arrow_forwardWhich of the following statements about AAS and AES is FALSE a)Sample used in AAS are usually in the form of aqueous solution b)In flame AAS, the flame is used for atomization as well as excitation purpose c)The graphite furnace is an example of non-flame atomizer d)In flame AES, no light source is required. What is the advantages of Axial torch configuration over Radial configuration in Inductive Couple Plasma-Optical Emission Spectrometer (ICP-OES) a)Increased radiation intensity – a longer path length b)Excellent tolerance to dissolve solids c)Can analyze S, P, and Halogens d)Excellent torch lifetimesarrow_forward
- Basic Clinical Lab Competencies for Respiratory C...NursingISBN:9781285244662Author:WhitePublisher:Cengage
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning




