
Concept explainers
(a)
Interpretation:
The reason behind less volatility of hexane than diethyl ether has to be described.
(a)
Explanation of Solution
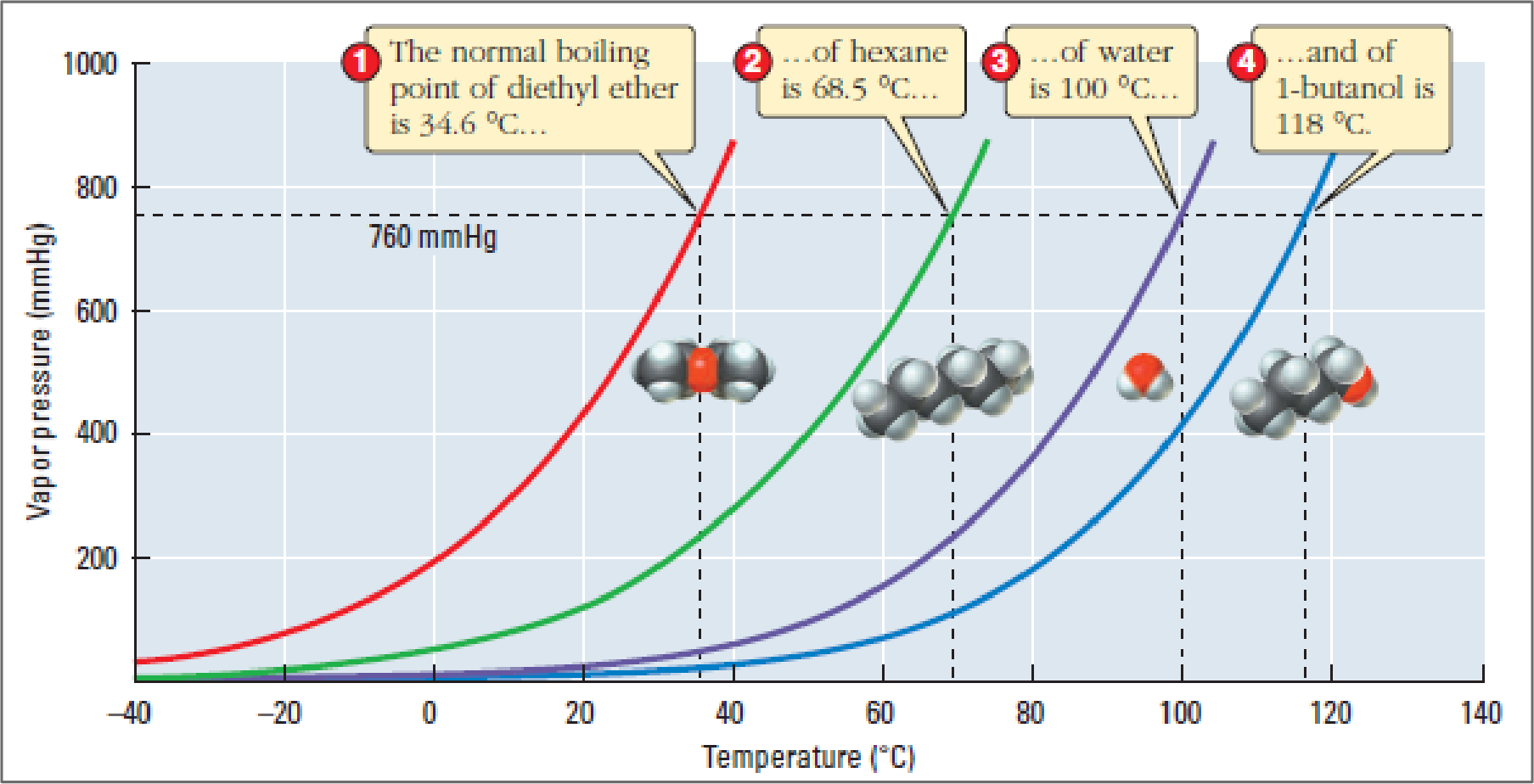
Figure 1
Vapour pressure of a liquid is defined as the pressure of the vapour when liquid and vapour are in dynamic equilibrium. It increases with increase in temperature and a liquid with stronger intermolecular force of attractions has a lower vapour pressure at a given temperature.
If a liquid is less volatile, then it must have a lower vapour pressure which can be accounted for its stronger intermolecular force of attraction. Examining the above figure, it can be concluded that hexane has a lower vapour pressure than diethyl ether at any temperature. The non-covalent intermolecular forces of attraction acting between the molecules of hexane is London forces while in diethyl ether they are dipole-dipole forces and London forces. Because liquid hexane has a lower vapour pressure, it can be predicted that the larger hexane molecules experience larger collective intermolecular London forces than diethyl ether.
So due to this reason, hexane is less volatile than diethyl ether.
(b)
Interpretation:
The temperature at which 1- butanol have a pressure of
(b)
Answer to Problem ISP
The temperature of 1-butanol at
Explanation of Solution
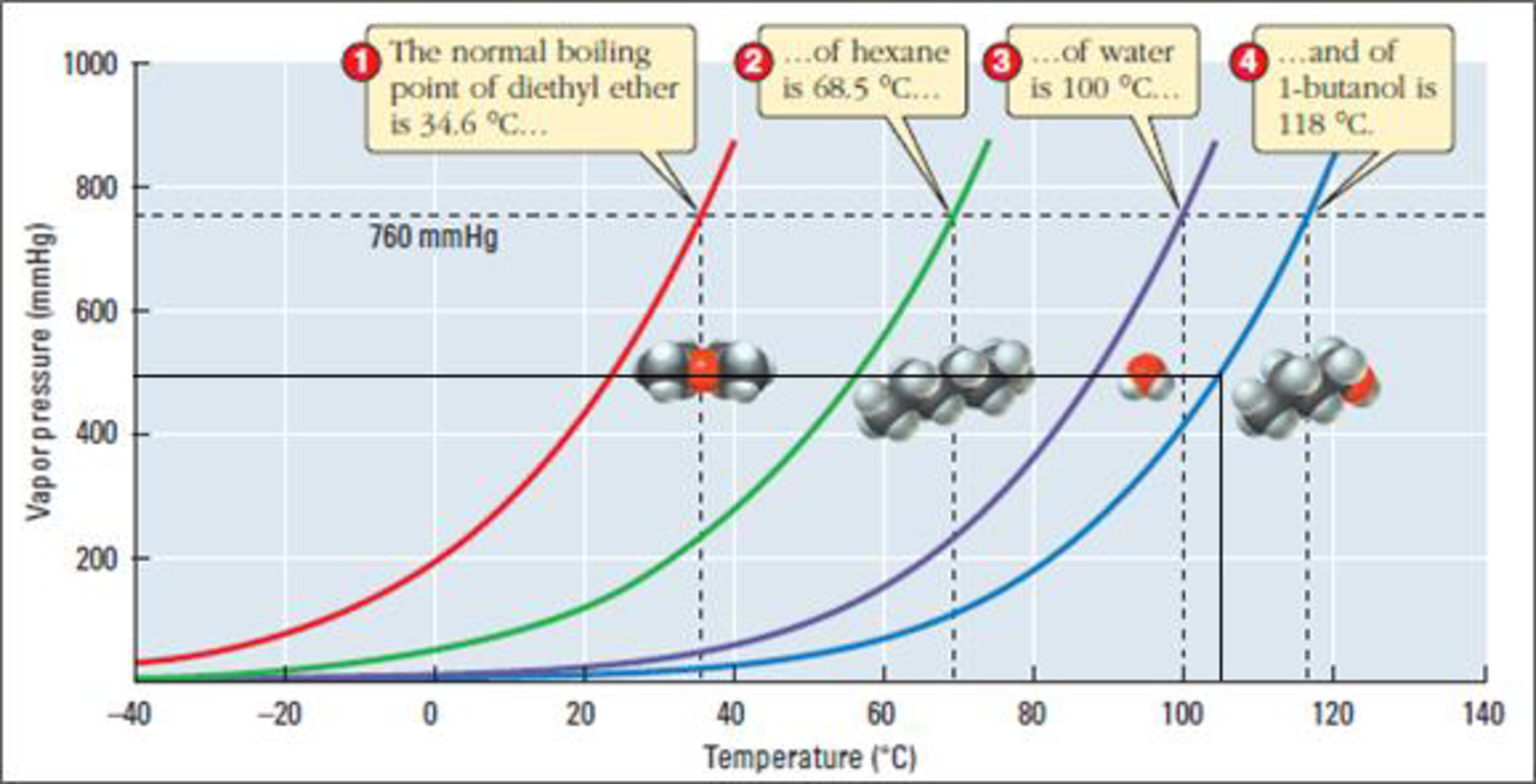
Figure 2
Examine the above figure carefully. Draw a horizontal line from
(c)
Interpretation:
The reason why the boiling point of 1-butanol is greater than that of water has to be described.
(c)
Explanation of Solution
The liquid having greater boiling point means it has stronger collective intermolecular forces. Both water and 1-butanol can experience hydrogen bonding interactions. Water is a small molecule with a total of
(c)
Interpretation:
The substance which would evaporate immediately and which would remain as liquid has to be determined.
(c)
Explanation of Solution
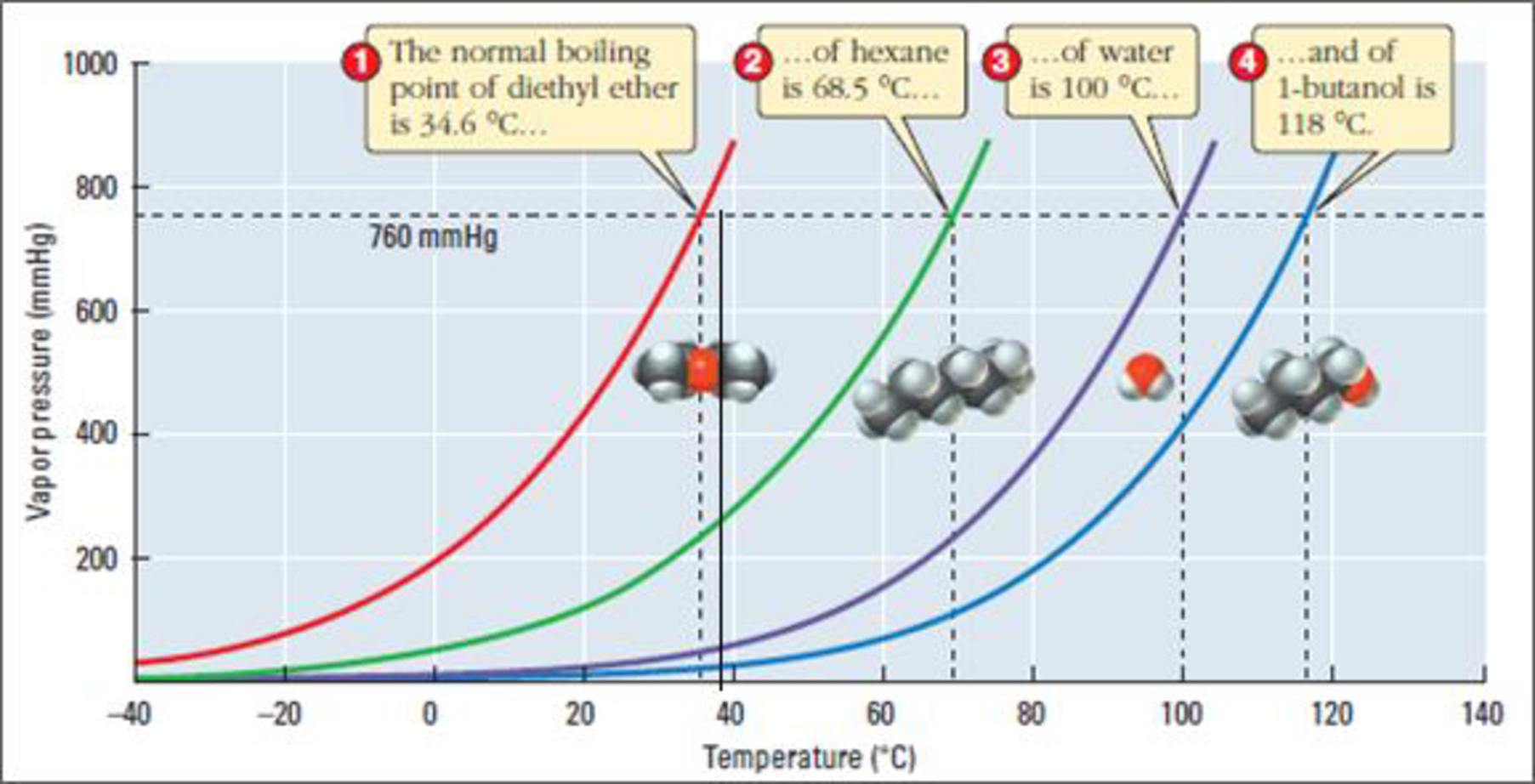
Figure 3
The boiling point of diethyl ether is
(d)
Interpretation:
The
Concept Introduction:
Clausius-Clapeyron equation:
Where,
P1 and P2 are two sets of pressures and T1 and T2 are two sets of absolute temperatures.
R is universal gas constant.
(d)
Answer to Problem ISP
The
Explanation of Solution
Given data:
The normal boiling point of 1-butanol is
Clausius-Clapeyron equation:
Substituting all the data in the above equation and solving for
Therefore, the
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: The Molecular Science
- Use molecular structures and noncovalent interactions to explain why dimethyl ether, (CH3)2O, is completely miscible in water, but dimethylsulfide, (CH3)2S, is only slightly water soluble.arrow_forward8.52 Rank the following hydrocarbons in order of increasing vapor pressure: C2H6,C10H22,CH4,C7H16,C22H46 .arrow_forwardA solution of benzoic acid in benzene has a freezing point of 3.1 C and a boiling point of 82.6 C. (The freezing point of pure benzene is 5.50 C, and its boiling point is 80.1 C) The structure of benzoic acid is Benzoic acid, C6H5CO2H What can you conclude about the state of the benzoic acid molecules at the two different temperatures? Recall the discussion of hydrogen bonding in Section 11.3.arrow_forward
- (a) 4. Identify the intermolecular forces which can operate between molecules of the following pure compounds: (b) N. (c) Numbering N. (d) 5. For the compounds in the previous question, what intermolecular forces would operate between the compound and water?arrow_forwardWhich of the following gases is expected to be most soluble in water? Explain your reasoning.(a) CH4(b) CCl4(c) CHCl3arrow_forwardWhich molecule ( CH3OH or CH4 ) has a greater vapor pressure and why?arrow_forward
- Why is hexane more volatile than water? Explain by mentioning the differences in their structure and intermolecular forces of attraction.arrow_forwardWhat intermolecular forces are in play for sodium stearate? Draw three water molecules; orienting them properly towards both sodium cation and stearate anion (label water molecules with crossed arrows, δ+ and δ- to indicate partial charges and polarityarrow_forwardWhy are the freezing and boiling points of water higher than would be expected for a compound of its molecular makeup?arrow_forward
- C. Explain the following occurrences in terms of molecular polarity and intermolecular forces. 1. Oils or fats cannot be removed by water alone. But oil can be removed when soap is used together with water. Structure of soap and fatty acid is shown below; (I did not show the structure of water because you know it already.) HH H Na HO-C-C- c-C-C-C-H HHHH HH Soap Fatty acidarrow_forwardAspirin has a higher molar mass compared to salicylic acid, however aspirin melts at a lower temperature than salicylic acid. Provide a brief explanation for this observation. Table 1 Compound: Formula: Salicylic Acid C;H6O3 Aspirin C9H3O4 Molar Mass: 138.12 Melting point: Ka 158-160°C 1.08 x 10³ 180.15 140-142°C 2.72 x 10$ pKa Solubility (g/100ML) 2.99 4.57 0.18 0.25arrow_forwardHow many grams of ethylene glycol (C2H6O2) must be added to 1000. g water to create anautomobile radiator coolant mixture that will not freeze at – 15⁰C?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





