
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.51SP
The compound shown below has three different types of OH groups, all with different acidities. Show the structure produced after this compound is treated with different amounts of NaH followed by a methylating reagent. Add a brief explanation.
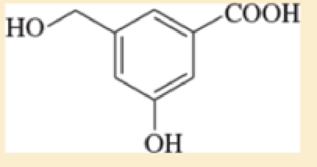
- a. 1 equivalent of NaH, followed by 1 equivalent of CH3I and heat
- b. 2 equivalents of NaH, followed by 2 equivalents of CH3I and heat
- c. 3 equivalents of NaH, followed by 3 equivalents of CH3I and heat
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
From the table of available reagents select the one(s) you would use to convert
1-phenylethanol to l-phenylethanethiol
Use the minimum number of steps; in no case are more than four steps necessary.
List reagents by letter in the order that they are used; example: fa.
Reagents available
.CO3H
a. CH3B.
e. H30*
i.
b. HCI
f. NaH
c. Hg(OCOCF3)2, (CH3)2C=CH2;
then NABH4
g. PBR3
h. POCI3, pyridine
j. (CH3)3COH
d. (H2N)2C=S; then "OH, H2O
How would you synthesize the following compounds from cyclohexanone
using reagents from the table?
Use letters from the table to list reagents in the order used (first at the left).
Reagents
a
1. CH3MgBr/dry ether
2 H3O+
e
1. OsO4
i
1. BH3/THF
2. NaHSO3/H2O
2. H₂O₂ / NaOH
b
1. C6H5MgBr / dry ether
f
1. NaBH4
j
Mg / dry ether
2 H3O+
2. H3O+
с
PBr3
g
HOCH2CH2OH/HCI
k
H2/Pd
d
m-chloroperbenzoic
h
H3O+ / heat
I
CrO3 / H3O+
acid
m
cyclohexanone
a) cyclohexylbenzene
b) 2-phenylcyclohexanone
Submit Answer
Try Another Version
3 item attempts remaining
How would you synthesize the following compounds from butanoic acid using reagents from the table?
Use letters from the table to list reagents in the order used (first at the left).
Example: ab
Reagents
1. NaBH4
PB13
KOH / alcohol
a
e
2. H30*
b
k
KOŁBU
1. LIAIH4
2. H30*
f
Mg / dry ether
H2/Pt
g
1. Li 2. Cul
1. СО2 2. НзО*
HBr, dark
no peroxides
d
h
1-bromobutane
m
1. BH3 / THF
2. H2O2 / NaOH
i
H3O*, heat
n
NaCN
a) octane
b) 2-bromobutane
Chapter 11 Solutions
Organic Chemistry (9th Edition)
Ch. 11.1 - Prob. 11.1PCh. 11.2C - Prob. 11.2PCh. 11.3 - Prob. 11.3PCh. 11.3 - Prob. 11.4PCh. 11.3 - Prob. 11.5PCh. 11.3 - Suggest the most appropriate method for each of...Ch. 11.4 - A chronic alcoholic requires a much larger dose of...Ch. 11.4 - Unlike ethylene glycol, propylene glycol...Ch. 11.5 - Predict the major products of the following...Ch. 11.5 - Show how you would convert propan-1-ol to the...
Ch. 11.6 - Predict the products of the following reactions....Ch. 11.7A - Propose a mechanism for the reaction of a....Ch. 11.7B - Prob. 11.13PCh. 11.7B - Show how you would use a simple chemical test to...Ch. 11.7C - Neopentyl alcohol, (CH3)3CCH2OH, reacts with...Ch. 11.7C - Prob. 11.16PCh. 11.7C - When cis-2-methylcyclohexanol reacts with the...Ch. 11.8 - Prob. 11.18PCh. 11.9 - Prob. 11.19PCh. 11.9 - Prob. 11.20PCh. 11.9 - Prob. 11.21PCh. 11.10A - Prob. 11.22PCh. 11.10A - Some alcohols undergo rearrangement or other...Ch. 11.10B - Prob. 11.24PCh. 11.10B - Explain why the acid-catalyzed condensation is a...Ch. 11.10B - Prob. 11.26PCh. 11.10B - When the following substituted cycloheptanol...Ch. 11.11A - Prob. 11.28PCh. 11.11A - Prob. 11.29PCh. 11.11B - Predict the products formed by periodic acid...Ch. 11.12 - Prob. 11.31PCh. 11.13A - Prob. 11.32PCh. 11.14 - Prob. 11.33PCh. 11.14 - a. Show how ethanol and cyclohexanol may be used...Ch. 11.14 - Prob. 11.35PCh. 11.14 - Phenols (pKa 10) are more acidic than other...Ch. 11.14 - To practice working through the early parts of a...Ch. 11.14 - Prob. 11.38PCh. 11 - Predict the major products of the following...Ch. 11 - Show how you would convert 2-methylcyclopentanol...Ch. 11 - In each case, show how you would synthesize the...Ch. 11 - Prob. 11.42SPCh. 11 - Prob. 11.43SPCh. 11 - Prob. 11.44SPCh. 11 - Both cis- and trans-2-methylcyclohexanol undergo...Ch. 11 - Prob. 11.46SPCh. 11 - Prob. 11.47SPCh. 11 - Show how you would make each compound, beginning...Ch. 11 - Predict the major products (including...Ch. 11 - Show how you would use simple chemical tests to...Ch. 11 - The compound shown below has three different types...Ch. 11 - Prob. 11.52SPCh. 11 - Prob. 11.53SPCh. 11 - Prob. 11.54SPCh. 11 - Prob. 11.55SPCh. 11 - Show how you would synthesize the following...Ch. 11 - Show how you would synthesize the following...Ch. 11 - The following pseudo-syntheses (guaranteed not to...Ch. 11 - Two unknowns, X and Y, both having the molecular...Ch. 11 - The Williamson ether synthesis involves the...Ch. 11 - Prob. 11.61SPCh. 11 - Prob. 11.62SPCh. 11 - Alcohols combine with ketones and aldehydes to...Ch. 11 - Prob. 11.64SPCh. 11 - Prob. 11.65SPCh. 11 - Prob. 11.66SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living By Chemistry: First Edition Textbook
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach (4th Edition)
Draw a Lewis structure for each covalent molecule. a. HBr b. CH3F c. H2O2 d. N2H4 e. C2H6 f. CH2Cl2
Principles of General, Organic, Biological Chemistry
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Design a synthetic route to demonstrate how you can convert 1-propanol into 2- hexyne. You must use 1-propanol as the source of all carbon atoms in the target molecule. Show all reagents needed and all molecules synthesized along the way. НО.arrow_forwardBe sure to answer all parts. An allylic alcohol contains an OH group on a carbon atom adjacent to a C-C double bond. Treatment of allylic alcohol A with HCl forms a mixture of two alllylic chlorides, B and C. Select a stepwise mechanism that illustrates how both products are formed. CI HCI + H20 HO. CI A B Part 1 out of 4 Select the correct first step. :Cl: он :Cl: HO. HO. :Cl: HO. HO. :Ci: :CI: HO. Next partarrow_forwardIdentify the reagent that is needed to complete the transformation shown below. Assume an acidic workup occurs if needed. HO но. Reagent OH ÓH A. Ag20, H20, NH40H O B. CrO3, H20, H2SO4 OC.LIAIH4 OD. NABH4 O E. Cro3, HCI, pyridinearrow_forward
- Identify the correct reagents and conditions to perform this chemoselective reaction: H ရှုံး H Select one: Me e. Reagent(s) and conditions? a. NaBH4 b. BH3 THF Pd/BaSO4, H₂ (1 atm.), quinoline, Pb(OAC)2, MeOH d. Pd/C, H2 (1 atm.), MeOH DIBAL Me H H OHarrow_forwardShow how to accomplish the following conversion using reagents from the table.arrow_forwardSelect the necessary reagent(s) for the following transformation.The answer is NOT NaOH a. NaOH b. Cu^2+ c. K2Cr2O7 d. H2/Pt e. I2 f. H+/H2Oarrow_forward
- HW Write a mechanism for the reaction shown below. Show all intermediates and use curved arrows to show electron flow. H3C 1. H₂SO4 cat. EtOH 2. Propose an efficient synthesis of the following compound. ???arrow_forwardHelp In finding the major organic products for these reactions + NaOCH2CH3 1. add slowly 2. HCI b) 1. NaOCH, CH3OH 2. (CH3)2CHCH2CH₂Br 3. H3O+, heatarrow_forwardThe compound shown below has three different types of OH groups, all with different acidities. Show the structureproduced after this compound is treated with different amounts of NaH followed by a methylating reagent. Add a briefexplanation. 3 equivalents of NaH, followed by 3 equivalents of CH3I and heatarrow_forward
- Consider the following alcohol, A. When reacted with one (or a combination of two) reagents in the "reagent box," A can be transformed into a variety of different molecules. H HO NaH: DMF POCI3 excess Nal DMSO SOBr₂ .Н PCI HO HO A Reagent Box 11 cat. H₂SO4 H₂O: acetone PBr3 Ho Hö List a reagent (or combination of two) from the reaction box capable of transforming A into the products given below. Note: there are many possible answers! Br POBr3 excess SOCI₂ НО TSCI CI OHarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. CN + H. 1. NaOH, heat 2. Neutralizing work-uparrow_forward16. Provide the best reagent(s) necessary to carry OH H20/heat b. NaOCH3 CH3CH2OH/heat d. Nacl in DMSO 17. Provide the best reagent(s) necessary to carry out the following conversion. OH Br a. NaBr in DMSO b. NAOH e. HBrarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY