
a)
Interpretation:
The expected product between the
Answer to Problem 21VC
The ethyl chloride undergoes SN2 reaction with Na+SCH3- and NaOH to yield a product.
The product is CH3CH2SCH3, CH3CH2OH.
Here the leaving group is Cl.
The nucleophile is SCH3-,OH.
Hence it undergoes SN2 reaction.
Explanation of Solution
The ethyl chloride undergoes SN2 reaction with Na+SCH3- and NaOH to yield a product.
The product is CH3CH2SCH3, CH3CH2OH.
Here the leaving group is Cl.
The nucleophile is SCH3-,OH.
Hence it undergoes SN2 reaction.
The ethyl chloride undergoes SN2 reaction with Na+SCH3- and NaOH to yield a product.
The product is CH3CH2SCH3, CH3CH2OH.
Here the leaving group is Cl.
The nucleophile is SCH3-,OH.
Hence it undergoes SN2 reaction.
b)
Interpretation:
The expected product between the alkyl halide CH3CH2Cl+Na+SCH3- and NaOH is interpreted.
Answer to Problem 21VC
The alkyl halide undergoes SN1 reaction with Na+SCH3- to yield a SCH3 Substituited product.
The product is shown in the reaction.
In this reaction leaving group is Cl, the nucleophile is SCH3-
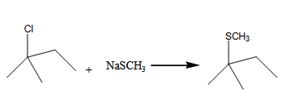
Explanation of Solution
The alkyl halide undergoes E1 reaction with NaOH to yield a
The product is shown here
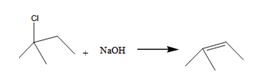
The alkyl halide undergoes SN1reaction with Na+SCH3- to yield a SCH3 Substituited product.
The product is shown in the reaction.
In this reaction leaving group is Cl, the nucleophile is SCH3-

c)
Interpretation:
The expected product between the alkyl halide CH3CH2Cl+Na+SCH3- and NaOH is interpreted.
Answer to Problem 21VC
The benzylchloride undergoes SN1 reaction with Na+SCH3- to yield a product.
The product is shown here.
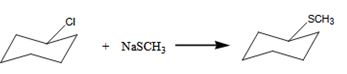
Here the leaving group is Cl.
The nucleophile is SCH3-Hence it undergoes SN1 reaction.
Explanation of Solution
The benzylchloride undergoes E1 reaction with NaOH to yield a product.
The product is shown here
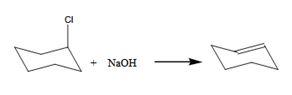
The benzylchloride undergoes SN1 reaction with Na+SCH3- to yield a product.
The product is shown here.
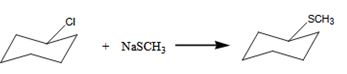
Here the leaving group is Cl.
The nucleophile is SCH3- Hence it undergoes SN1 reaction.
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
- When 2-iodo-1,4-dimethylcyclohexane is heated in acetic acid, CH3COOH, a mixture of substitution and elimination products is obtained. Provide structures for all possible products, writing [not drawing] the name of the mechanism by which each one is formed.arrow_forwardWrite the appropriate reagents, conditions and products for the following transformations, in a single step. OH II HNO, ? (1) H,SO,arrow_forwardPredict the product obtained when 1-pentyne reacts with an excess of HBr:arrow_forward
- Predict (by name) the major product for the following reaction. The starting compound is byblo[4.4.0]dec-1-ene. Note the numbering and name the product.arrow_forwardIf phenoxide ion is allowed to react with 1-bromopentane, pentyl phenyl ether is obtained. However, if cyclohexane is used as the alkyl halide, the major products are phenol and cyclohexene. Explain how these products were formed.arrow_forwardProvide the structure(s) of the expected major organic product of the reaction shown. 1) Disiamylborane 2) H₂O₂, NaOH OI O II ||| O IV OV CH3CH₂C(CH3)2C=CH OH OH xx xo IVarrow_forward
- Alkylation of benzene with 1-chlorobutane in the presence of AlCl3 gave not only the expected butylbenzene product but also, as a major product, (1-methylpropyl)benzene. Write an equation for the reaction Propose a mechanism to account for the formation of butylbenzene Propose a mechanism to account for the formation of (1-methylpropyl)benzenearrow_forwardFollowing are two diastereomers of 3-bromo-3,4-dimethylhexane. On treatment with sodium ethoxide in ethanol, each gives 3,4-dimethyl-3-hexene as the major product. One diastereomer gives the E alkene, and the other gives the Z alkene. Which diastereomer gives the Z alkene? Et Me Et Et Et Br Me H Ме b H Br Me aarrow_forwardWhich functional group(s) would be added to 1-methylcyclohexene using the reagents below: H3O+ hydrogen hydroxyl ketone and aldehyde bromine and hydroxyl bromine and hydrogen bromine hydrogen and hydroxyl aldehyde ketone Save for Laterarrow_forward
- The reaction of methylpropene with HBr, under radical conditions, gives two intermediates. Propose a mechanism for the formation of the two products. Propose a mechanism for the following reaction and use electronic factors to account for the formation of a major product: CH2 CH2Br N-Bromosuccinimide (NBS) ho, CCI4 Draw the structure of an antioxidant, Vitamin E free radical and use resonance structures o account for its stability.arrow_forwardWhat products would you expect from reaction of the following alkenes with NBS? If more than one product is formed, show the structures of all.arrow_forwardWrite a mechanism that accounts for the formation of ethyl isopropyl ether as one of the products in the following reaction. CI OEt HCI EtOH Write the mechanism for step one of this reaction. Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Write the mechanism for step two of this reaction (where the product of step one reacts with the solvent, ethanol). Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Write the mechanism for the last step of this reaction (formation of ethyl isopropyl ether). Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. CI will act as the base in this reaction.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

