
Concept explainers
(a)
Interpretation:
From the condensed structures for 2,3-dimethyl-2-pentene whether cis-trans isomers exist or not has to be given. And if it exists, both the isomers has to be drawn.
Concept Introduction:
Condensed structures: A condensed structures only shows the total number of atoms but not bonds exist between the atoms. The vertical and horizontal line which represents bonding all should be omitted.
Line structure: In organic compounds, the series of atoms in the compound are bonded together which shown by drawing a line between them. End of line segment represents carbon
Predicting cis-trans isomers:
In general, the
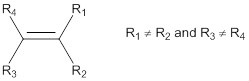
Cis-isomer: In cis-configuration, the two bulkier substituents of the double bond are on the same side.
Trans-isomer: In trans-configuration, the two bulkier substituents of the double bond are on the opposite side.
(b)
Interpretation:
From the both condensed and line structures for 2-Methyl-2-hexene whether cis-trans isomers exist or not has to be given. And if it exists, both the isomers has to be drawn.
Concept Introduction:
Condensed structures: A condensed structures only shows the total number of atoms but not bonds exist between the atoms. The vertical and horizontal line which represents bonding all should be omitted.
Line structure: In organic compounds, the series of atoms in the compound are bonded together which shown by drawing a line between them. End of line segment represents carbon
Cis-isomer: In cis-configuration, the two bulkier substituents of the double bond are on the same side.
Trans-isomer: In trans-configuration, the two bulkier substituents of the double bond are on the opposite side.
(c)
Interpretation:
From the line structures for 2-hexene whether cis-trans isomers exist or not has to be given. And if it exists, both the isomers has to be drawn.
Concept Introduction:
Condensed structures: A condensed structures only shows the total number of atoms but not bonds exist between the atoms. The vertical and horizontal line which represents bonding all should be omitted.
Line structure: In organic compounds, the series of atoms in the compound are bonded together which shown by drawing a line between them. End of line segment represents carbon
Cis-isomer: In cis-configuration, the two bulkier substituents of the double bond are on the same side.
Trans-isomer: In trans-configuration, the two bulkier substituents of the double bond are on the opposite side.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- Drawn are four isomeric dimethylcyclopropanes. How are the compounds in each pair related (enantiomers, diastereomers, constitutional isomers): A and B; A and C; B and C; C and D?arrow_forwardWrite chemical names for the following compounds: (a) Thymidine (b) Cytosine (c) Uracil (d) Xanthine (e) Guanine () 2,4-dioxy-6-carboxy pyrimidine (g) CAMP (h) dTTParrow_forwardWhen Alfred Werner was developing the field of coordination chemistry, it was argued by some that the optical activity he observed in the chiral complexes he had prepared was due to the presence of carbon atoms in themolecule. To disprove this argument, Werner synthesized a chiral complex of cobalt that had no carbon atoms in it, and he was able to resolve it into its enantiomers. Design a cobalt(III) complex that would be chiral if it could be synthesized and that contains no carbon atoms.arrow_forward
- (A) What are Waxes? Draw the structure of wax, which is made up of palmitic acid (16:0) and a saturated 18-carbon alcohol. (В) Consider the structure of menthol. How many isoprene units are present in menthol? (C) What type of isoprene linkage (head-to-tail or tail-to-tail) is present in menthol? Identify the isoprene linkage in the following structure of menthol and indicate by a circle. Menthol =arrow_forwardDraw the structures of the following compounds. (Includes both new and old names.) 3-cyclopentylhexan-3-olarrow_forwardThe explosive trinitrotoluene (TNT) is made by carrying out three successive nitration reactions on toluene. If these nitrations only occur in the ortho and para positions relative to the methyl group, what is the structure of TNT?arrow_forward
- Draw structural formulas for all of the following. Q.) Alcohols with the molecular formula C4H10Oarrow_forwardWhich of the following define the stereochemistry of alanine (as per the structure shown)? Note: Functional groups arranged horizontally are facing towards the front, and the functional groups arranged vertically are facing towards the back. СООН + H₂N to OS- Od- CH, OR-arrow_forwardH OH CH2OH D H ОН H الحزن ОН CH2OH ОН H ОН I Which of the following statements correctly describes this structure? (A) The monomer units are bonded by beta 1-2 glycosidic linkage. B The monomer units are bonded by alpha 1-2 glycosidic linkage. The monomer units are bonded by alpha 1-4 glycosidic linkage. The monomer units are bonded by beta 1-4 glycosidic linkage. H CH2OH H ОН H ОН H ОНarrow_forward
- Compound A is a dipeptide, optically inactive. While compound B is a tripeptide, and optically active. Compound A is formed when compound C and compound D joined together by condensation reaction. Whereas monomers E and F are formed by modifying the compounds C and D. Polymer G is formed by the condensation reaction of monomers E and F. Draw the possible structural formulae A, B,C,D,E,F and polymer G. Label the peptide bond(s) for compounds A and B. Pls name the compounds explain tooarrow_forwardCompound A undergoes a reaction with hydrogen bromide, HBr to produce2-bromobutane. A exists as cis-trans isomers and decolourises brominesolution in methylene chloride, CH2Cl2. a)Draw and name the structure of compound D. b)Draw two (2) constitutional isomers of compound Darrow_forwardSpermaceti, a fragrant substance isolated from sperm whales, was commonly used in cosmetics until it was banned in 1976 to protect the whales from extinction. Chemically, spermaceti is cetyl palmitate, the ester of palmiticacid with cetyl alcohol (the straight-chain 16-carbon alcohol). Draw the structure of spermaceti.arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





