
Concept explainers
Interpretation:
The
Concept introduction:
Phenylalanine is an α-amino acid with the formula Phenylalanine is an α-amino acid with the formula C9H11NO2.
Here, the benzyl group is substituted for the methyl group of alanine, or a phenyl group in place of terminal hydrogen of alanine.
Lidocaine, also known as xylocaine and lignocaine, is a medication used to numb tissue in a specific area.
Formula: C14H22N2O
Answer to Problem 19VC
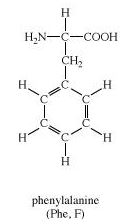
Here, Primary
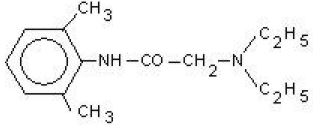
Here, amide group is present as functional group in this molecule.
Explanation of Solution
From the structure of phenylalanine, it is quite evident that the terminal hydrogen of alanine is replaced by a benzyl or phenyl group.
In this way, carbon forms a covalent bond with another carbon atom or other atoms.
-NH2 group and –COOH groups are present in Phenylalanine.
-NH and C=O (Amide) groups are present as functional groups in Lidocaine.
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry
- Formaldehyde, H2C=O, is known to all biologists because of its usefulness as a tissue preservative. When pure, formaldehyde trimerizes to give trioxane, C3H6O3, which, surprisingly enough, has no carbonyl groups. Only one monobromo derivative (C3H5BrO3) of trioxane is possible. Propose a structure for trioxane.arrow_forwardDraw a skeletal ("line") structure of this molecule: CH3 OH CH3 CH2-C=C-CH3 Click and drag to start drawing a structure. с с Ś cx Ćarrow_forwardYour chemistry professor draws a number of molecules on the board: (1) CH4 ; (2) H2C=CH2 ; (3) H2C=C=CH2 ; and (4) H2C=C=C=CH2. You muse about all the molecules that chemists draw on a two-dimensional board and wonder which ones are actually planar, existing basically as they appear on the board, and which ones are not plane but rather three-dimensional. Answer the following questions. What is the geometry and hybridization of the carbon in CH4? What is the geometry and hybridization of each central carbon atom in the remaining molecules? Draw each molecule showing the bonds and identify each bond in all the molecules as s or p. What are the specific orbital overlaps (i.e., sp3-sp3) that are in each of the molecules? What are the bond angles for each central atom in each molecule? Which molecules are planar and which are non-planar?arrow_forward
- Petroleum is a mixture of hydrocarbons made out of decay microscopic ocean-dwelling plants and animals. Crude oil which is the product collects in underground pockets in sedimentary rock can be separated to different fractions by fractional distillation. As a chemical engineer, you are required to prepare the specific details of the products. Two fractions are produced from molecular range C1-C4 and C5 - C12. For each molecular range write the structures for 5 different compounds, formulas and also contain functional group for the IUPAC name of each compound and then identify 2 different functional groups among the structures. Next, provide 5 structures using the dash format, condensed format, bond-line format and full three-dimensional format. Describe the physical properties of the 5 compounds . Determine the rank in increasing order and explain on the basis of intermolecular forces and polarity.arrow_forwardDraw an expanded structural formula of pent-1-en-3-yne/ CH3-CC-CH=CH2 and then label each carbon. Indicate the longest and shortest C-H bond and predict the C—C single bond that has the highest BDE(bond dissociation energy).arrow_forwardChoose the correct answer. Choose. Choose. An alkyne with molecular formula C5H10 A ketone with molecular formula C4H80 An aldehyde with molecular formula C2H4O A saturated hydrocarbon with molecular formula C6H14 An alkene with molecular formula C5H10 An alkene with molecular formula C5H8 A ketone with molecular formula C3H8O An aldehyde with molecular formula CH40 Choose.arrow_forward
- Compound A, C4H40, undergoes the ring substitution reactions characteristic of aromatics to give two isomers. Draw the structure of A. ring substitution 2 substitution isomers A Draw cations and anions in separate sketchers. • Separate structures with + signs from the drop-down menu. ChemDoodlearrow_forwardIdentify the polar covalent bond and write the charge &+/ &- нно T T | Н-С-Ҫ-С-Н ÇI нон H-C-H Н-С-ОН Н-С-С-С-Н нн chloromethane, CH,CI methanol propanal propanonearrow_forwardWhich line structures represent the molecule in the box? 1110 애 Br (A 오 三 (E 애 애 111 Br Br H Br B B- F Br 애 애 애 Brarrow_forward
- Which of the following structures, including formal charges, is correct for diazomethane? H₂CNN: H₂C=N=N: H₂C N3 EN: H₂C=N= EN: +3 -3 7:2: H₂C-N- N:arrow_forwardName the following hydrocarbons: H (a) H2C=C=C-CH,-CH,-CH3 нн (b) H2C=C-C=C-C=CH2 нн CH3 (c) Н.С—С-СH —CH,—CH—CH, (d) CH;-CH2-C=C-CH2-CH;arrow_forwardEstimate the heat released when ethene(CH2=CH2) reacts with HBr to giveCH3CH2Br. Bond enthalpies areC-H : 412 kJ/mol; C-C : 348 kJ/mol;C=C : 612 kJ/mol; C-Br : 276 kJ/mol;Br-Br : 193 kJ/mol; H-Br : 366 kJ/mol. Choose the correct answer:1. 1036 kJ/mol2. 58 kJ/mol3. 424 kJ/mol4. 200 kJ/mol5. 470 kJ/molarrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


